Abstract
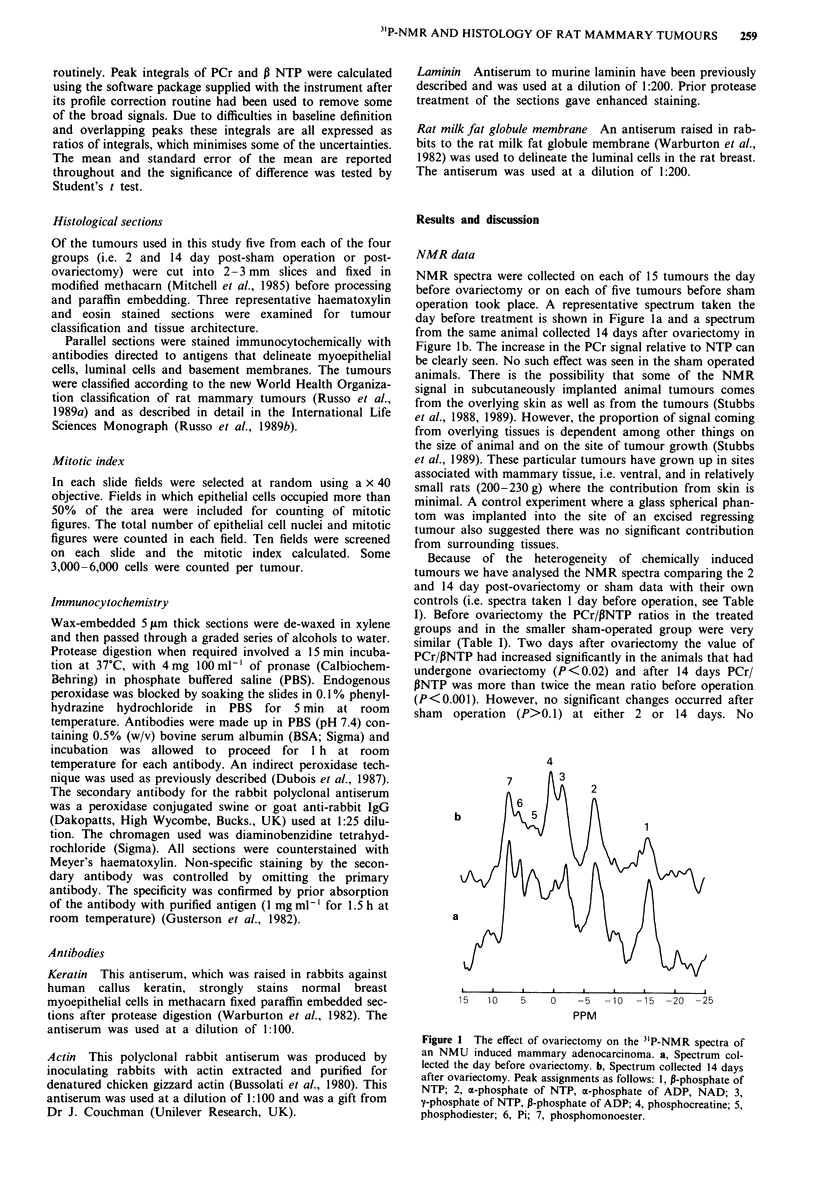
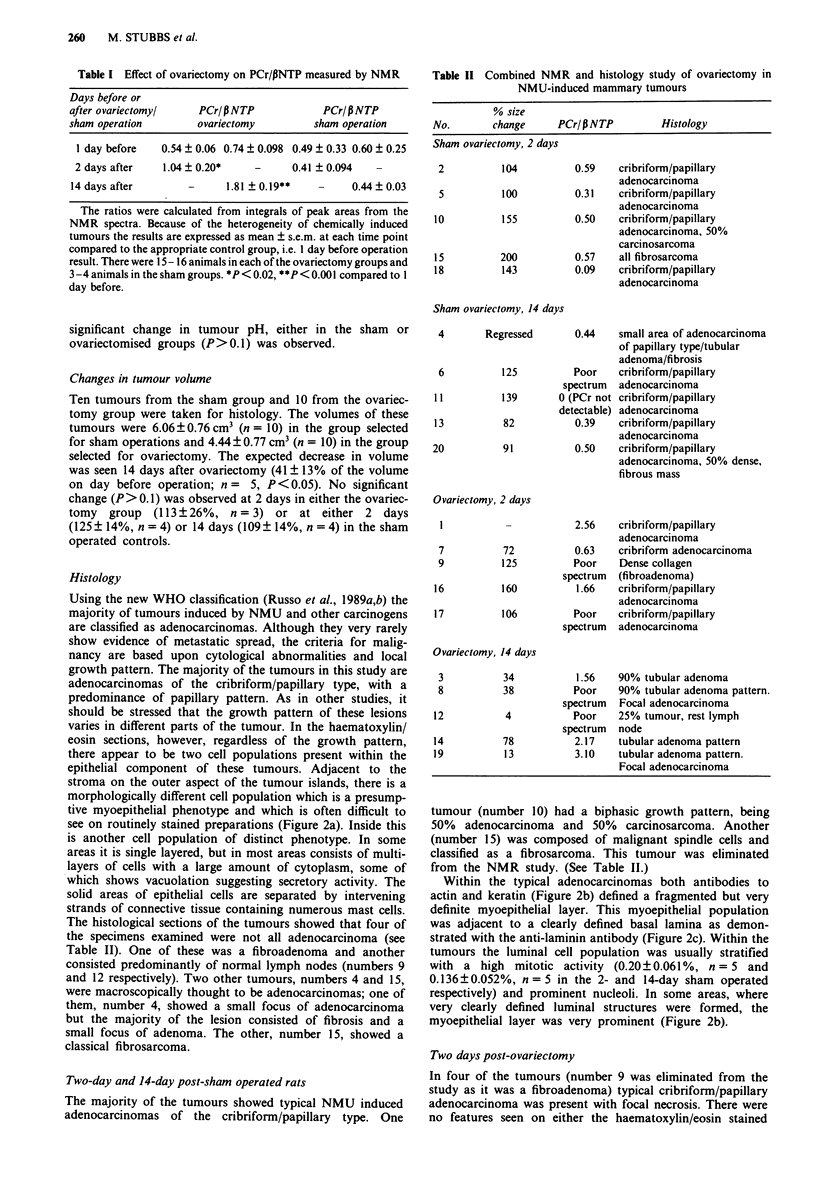
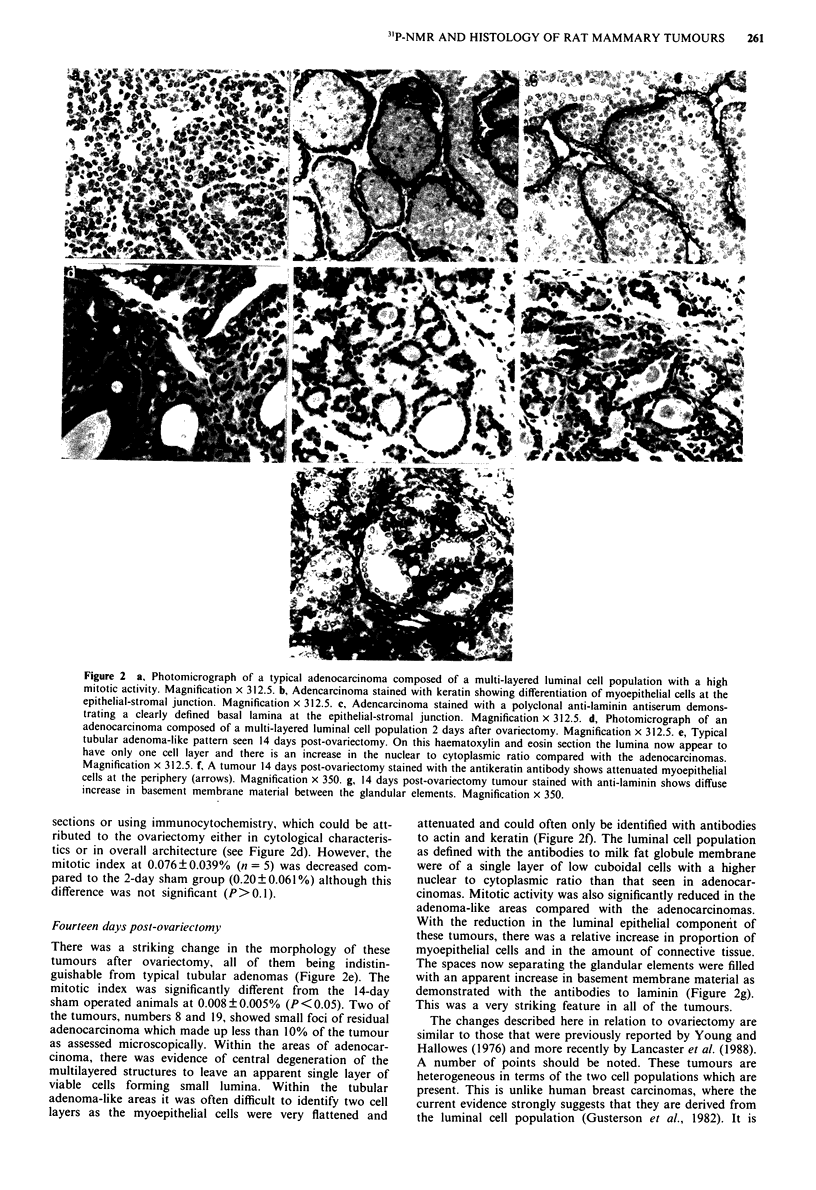
We have shown by 31P-NMR spectroscopy that ovariectomy, in N-methyl-N-nitrosourea induced mammary adenocarcinomas, increases signals from phosphocreatine (PCr) relative to nucleoside triphosphate (NTP) before measurable regression (2 days) and for at least a further 13 days. The present study correlates the NMR changes with histological changes in the regressing tumour. Mammary tumours were examined by NMR before, and 2 and 14 days after, ovariectomy or sham-ovariectomy. Sections were taken from five tumours at each time point after operation for histology and for immunocytochemical staining of myoepithelial cells, luminal cells and basement membrane material. The histology showed typical cribriform papillary type mammary adenocarcinomas. The luminal cell population had a high mitotic activity and there was a prominent myoepithelial layer. At 2 days post-ovariectomy no significant change in mitotic activity was observed and no cytological characteristics attributable to ovariectomy could be seen. At 14 days postovariectomy the tumour was indistinguishable from a tubular adenoma, had significantly reduced mitotic activity, a relative increase in myoepithelial cells and basement membrane material. The changes detected by NMR must reflect early metabolic events, perhaps related to the histological changes observed at 14 days after ovariectomy. 31P-NMR spectroscopy may permit early monitoring of endocrine therapy for mammary cancer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Bussolati G., Alfani V., Weber K., Osborn M. Immunocytochemical detection of actin on fixed and embedded tissues: its potential use in routine pathology. J Histochem Cytochem. 1980 Feb;28(2):169–173. doi: 10.1177/28.2.6986431. [DOI] [PubMed] [Google Scholar]
- Dubois J. D., O'Hare M. J., Monaghan P., Bartek J., Norris R., Gusterson B. A. Human breast epithelial xenografts: an immunocytochemical and ultrastructural study of differentiation and lactogenic response. Differentiation. 1987;35(1):72–82. doi: 10.1111/j.1432-0436.1987.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Evanochko W. T., Ng T. C., Glickson J. D. Application of in vivo NMR spectroscopy to cancer. Magn Reson Med. 1984 Dec;1(4):508–534. doi: 10.1002/mrm.1910010410. [DOI] [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Ellison M., Neville A. M., Rudland P. S. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982 Nov;42(11):4763–4770. [PubMed] [Google Scholar]
- Lancaster S., English H. F., Demers L. M., Manni A. Kinetic and morphometric responses of heterogeneous populations of experimental breast cancer cells in vivo. Cancer Res. 1988 Jun 1;48(11):3276–3281. [PubMed] [Google Scholar]
- Mitchell D., Ibrahim S., Gusterson B. A. Improved immunohistochemical localization of tissue antigens using modified methacarn fixation. J Histochem Cytochem. 1985 May;33(5):491–495. doi: 10.1177/33.5.3921605. [DOI] [PubMed] [Google Scholar]
- Rodrigues L. M., Midwood C. J., Coombes R. C., Stevens A. N., Stubbs M., Griffiths J. R. 31P-nuclear magnetic resonance spectroscopy studies of the response of rat mammary tumors to endocrine therapy. Cancer Res. 1988 Jan 1;48(1):89–93. [PubMed] [Google Scholar]
- Steen R. G., Tamargo R. J., McGovern K. A., Rajan S. S., Brem H., Wehrle J. P., Glickson J. D. In vivo 31P nuclear magnetic resonance spectroscopy of subcutaneous 9L gliosarcoma: effects of tumor growth and treatment with 1,3-bis(2-chloroethyl)-1-nitrosourea on tumor bioenergetics and histology. Cancer Res. 1988 Feb 1;48(3):676–681. [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L. M., Griffiths J. R. Potential artefacts from overlying tissues in 31P NMR spectra of subcutaneously implanted rat tumours. NMR Biomed. 1989 Apr;1(4):165–170. doi: 10.1002/nbm.1940010403. [DOI] [PubMed] [Google Scholar]
- Stubbs M., Vanstapel F., Rodrigues L. M., Griffiths J. R. Phosphate metabolites in rat skin. NMR Biomed. 1988 Feb;1(1):50–55. doi: 10.1002/nbm.1940010109. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Bhujwalla Z. M., Griffiths J. R., Maxwell R. J. Phosphorus-31 magnetic resonance spectroscopy and blood perfusion of the RIF-1 tumor following X-irradiation. Int J Radiat Oncol Biol Phys. 1989 Jan;16(1):155–164. doi: 10.1016/0360-3016(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Mitchell D., Ormerod E. J., Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982 Jul;30(7):667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Gusterson B., Humphreys J., Monaghan P., Coombes R. C., Rudland P., Neville A. M. N-methyl-N-nitrosourea-induced rat mammary tumors. Hormone responsiveness but lack of spontaneous metastasis. J Natl Cancer Inst. 1981 Jan;66(1):147–155. [PubMed] [Google Scholar]



