Abstract
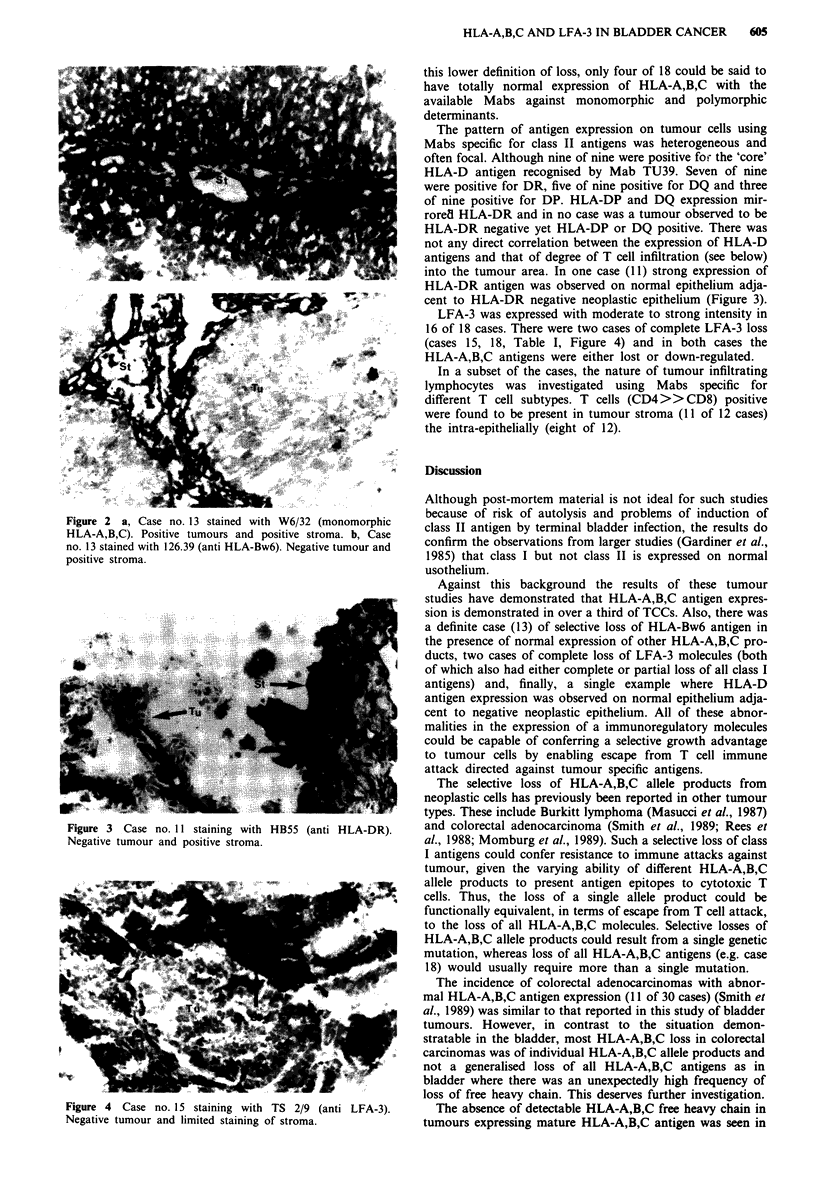
The expression of the major histocompatibility complex (MHC) class I and II antigens and lymphocyte function-associated antigen-3 (LFA-3) was investigated using immunohistochemical staining of bladder tissue sections from 18 patients with transitional cell carcinoma (TCC) and two normal bladder specimens. The expressions of HLA-A,B,C antigens varied greatly between different tumours. Complete loss was observed in one of 18 cases. Moderate to strong expression of HLA-A,B,C antigens was observed in 10 of 18 cases with the remaining seven cases showing either weak expression or expression on only a proportion of the tumour cells. Selective loss of HLA-Bw6 was seen in one of 18 cases. In many cases heterogenous and often focal expression of HLA-D products was seen. In one case tumour cells not expressing HLA-DR antigens were adjacent to strongly HLA-DR expressing non-neoplastic bladder epithelium, indicating a lack of inducible HLA-DR in the tumour cells. LFA-3 was undetectable in two of 18 cases with the remaining 16 cases showing moderate to strong expression of the molecule. These findings indicate that a substantial proportion of bladder tumours have one or more of a wide range of different alterations in the expressions of immunoregulatory molecules that could contribute to escape from immune surveillance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger A. E., Davis J. E., Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1(2):87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. J., Lotze M. T., Roberts J. R., Rosenberg S. A., Jaffe E. S. The immunopathology of sequential tumor biopsies in patients treated with interleukin-2. Correlation of response with T-cell infiltration and HLA-DR expression. Am J Pathol. 1987 Nov;129(2):208–216. [PMC free article] [PubMed] [Google Scholar]
- Festenstein H., Schmidt W. Variation in MHC antigenic profiles of tumor cells and its biological effects. Immunol Rev. 1981;60:85–127. doi: 10.1111/j.1600-065x.1981.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Fleming K. A., McMichael A., Morton J. A., Woods J., McGee J. O. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981 Jul;34(7):779–784. doi: 10.1136/jcp.34.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner R. A., Seymour G. J., Lavin M. F., Strutton G. M., Gemmell E., Hazan G. Immunohistochemical analysis of the human bladder. Br J Urol. 1986 Feb;58(1):19–25. doi: 10.1111/j.1464-410x.1986.tb05420.x. [DOI] [PubMed] [Google Scholar]
- Gotch F., Rothbard J., Howland K., Townsend A., McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. 1987 Apr 30-May 6Nature. 326(6116):881–882. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- Gregory C. D., Murray R. J., Edwards C. F., Rickinson A. B. Downregulation of cell adhesion molecules LFA-3 and ICAM-1 in Epstein-Barr virus-positive Burkitt's lymphoma underlies tumor cell escape from virus-specific T cell surveillance. J Exp Med. 1988 Jun 1;167(6):1811–1824. doi: 10.1084/jem.167.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K., Grosveld F., Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984 Oct 25;311(5988):750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- Krensky A. M., Robbins E., Springer T. A., Burakoff S. J. LFA-1, LFA-2, and LFA-3 antigens are involved in CTL-target conjugation. J Immunol. 1984 May;132(5):2180–2182. [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Masucci M. G., Torsteindottir S., Colombani J., Brautbar C., Klein E., Klein G. Down-regulation of class I HLA antigens and of the Epstein-Barr virus-encoded latent membrane protein in Burkitt lymphoma lines. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4567–4571. doi: 10.1073/pnas.84.13.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Rothbard J. HLA B37 determines an influenza A virus nucleoprotein epitope recognized by cytotoxic T lymphocytes. J Exp Med. 1986 Nov 1;164(5):1397–1406. doi: 10.1084/jem.164.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momburg F., Degener T., Bacchus E., Moldenhauer G., Hämmerling G. J., Möller P. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int J Cancer. 1986 Feb 15;37(2):179–184. doi: 10.1002/ijc.2910370203. [DOI] [PubMed] [Google Scholar]
- Momburg F., Ziegler A., Harpprecht J., Möller P., Moldenhauer G., Hämmerling G. J. Selective loss of HLA-A or HLA-B antigen expression in colon carcinoma. J Immunol. 1989 Jan 1;142(1):352–358. [PubMed] [Google Scholar]
- Pawelec G. P., Shaw S., Ziegler A., Müller C., Wernet P. Differential inhibition of HLA-D- or SB-directed secondary lymphoproliferative responses with monoclonal antibodies detecting human Ia-like determinants. J Immunol. 1982 Sep;129(3):1070–1075. [PubMed] [Google Scholar]
- Rees R. C., Buckle A. M., Gelsthorpe K., James V., Potter C. W., Rogers K., Jacob G. Loss of polymorphic A and B locus HLA antigens in colon carcinoma. Br J Cancer. 1988 Apr;57(4):374–377. doi: 10.1038/bjc.1988.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. E., Bodmer W. F., Bodmer J. G. Selective loss of HLA-A,B,C locus products in colorectal adenocarcinoma. Lancet. 1988 Apr 9;1(8589):823–824. doi: 10.1016/s0140-6736(88)91682-0. [DOI] [PubMed] [Google Scholar]
- Smith M. E., Marsh S. G., Bodmer J. G., Gelsthorpe K., Bodmer W. F. Loss of HLA-A,B,C allele products and lymphocyte function-associated antigen 3 in colorectal neoplasia. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5557–5561. doi: 10.1073/pnas.86.14.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Borst J., Giphart M., Coligan J., Terhorst C., De Vries J. E. HLA-DC antigens can serve as recognition elements for human cytotoxic T lymphocytes. Eur J Immunol. 1984 Apr;14(4):299–304. doi: 10.1002/eji.1830140404. [DOI] [PubMed] [Google Scholar]
- Stam N. J., Spits H., Ploegh H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986 Oct 1;137(7):2299–2306. [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Watson A. J., DeMars R., Trowbridge I. S., Bach F. H. Detection of a novel human class II HLA antigen. 1983 Jul 28-Aug 3Nature. 304(5924):358–361. doi: 10.1038/304358a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]