Abstract
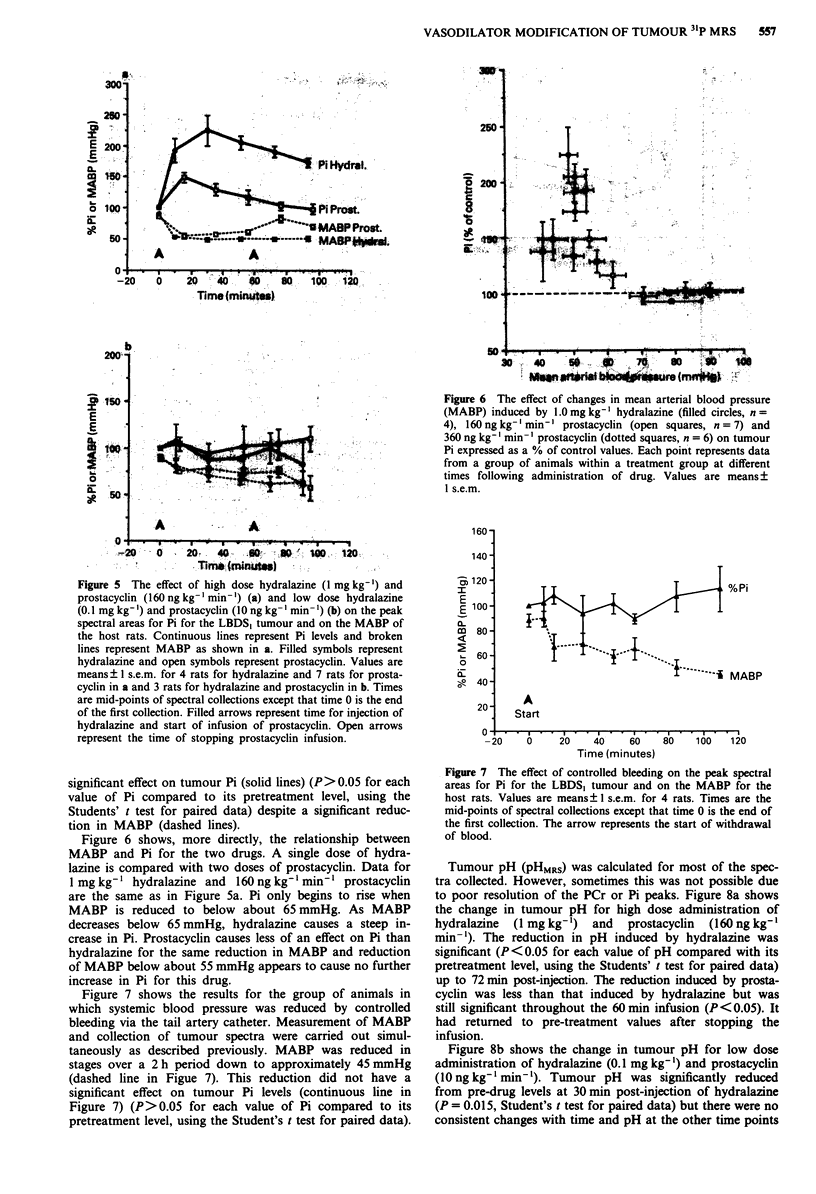
The effects of different doses of hydralazine and prostacyclin on the 31P magnetic resonance spectra of the LBDS1 fibrosarcoma were investigated and related to their effects on mean arterial blood pressure (MABP) and heart rate. The effect of reducing MABP by bleeding the animals, via the tail artery, was also investigated. Tumour spectral changes following high dose drug treatment (an increase in inorganic phosphate, a reduction in nucleotide triphosphates and a reduction in pH) were consistent with nutrient deprivation. These changes were dose dependent. Changes in MABP and heart rate were consistent with vasodilatation in normal tissues. However, for the same fall in MABP, hydralazine produced a greater rise in tumour inorganic phosphate (Pi) and a greater fall in tumour pH than did prostacyclin. Controlled bleeding was effective in reducing MABP. It also reduced tumour pH but had no significant effect on tumour Pi. The clinical application of the two drugs for reducing tumour blood flow and pH for therapy is likely to be limited by the large degree of hypotension necessary to produce an effect. The differential effect of the two drugs for the same fall in MABP may be related to different degrees of direct tumour vasodilatation or to a direct effect of hydralazine on tumour energy metabolism. The observation that controlled bleeding does not change tumour Pi is further evidence indicating that the degree of arterial hypotension is not the sole factor in determining tumour energy status.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. M., Chapple D., Dusting G. J., Hughes R., Moncada S., Vane J. R. Cardiovascular actions of prostacyclin (PCI2) in chloralose anaesthetized dogs [proceedings]. Br J Pharmacol. 1977 Sep;61(1):136P–136P. [PMC free article] [PubMed] [Google Scholar]
- Babbs C. F., DeWitt D. P., Voorhees W. D., McCaw J. S., Chan R. C. Theoretical feasibility of vasodilator-enhanced local tumor heating. Eur J Cancer Clin Oncol. 1982 Nov;18(11):1137–1146. doi: 10.1016/0277-5379(82)90095-5. [DOI] [PubMed] [Google Scholar]
- Chan R. C., Babbs C. F., Vetter R. J., Lamar C. H. Abnormal response of tumor vasculature to vasoactive drugs. J Natl Cancer Inst. 1984 Jan;72(1):145–150. doi: 10.1093/jnci/72.1.145. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Chiavarelli M., Moncada S., Mullane K. M. Prostacyclin can either increase or decrease heart rate depending on the basal state. Br J Pharmacol. 1982 Jan;75(1):243–249. doi: 10.1111/j.1476-5381.1982.tb08779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLASS C. D., DILLAHA C. J., DILLAHA J., KOUNTZ S. L. Inhibition of biologic acetylation by 1-hydrazinophthalazine. J Lab Clin Med. 1957 Apr;49(4):561–565. [PubMed] [Google Scholar]
- Dunn J. F., Frostick S., Adams G. E., Stratford I. J., Howells N., Hogan G., Radda G. K. Induction of tumour hypoxia by a vasoactive agent. A combined NMR and radiobiological study. FEBS Lett. 1989 Jun 5;249(2):343–347. doi: 10.1016/0014-5793(89)80655-6. [DOI] [PubMed] [Google Scholar]
- Falk P. The angio-architecture of rat tumours. Bibl Anat. 1977;(15 Pt 1):245–248. [PubMed] [Google Scholar]
- Hahn G. M., Shiu E. C. Adaptation to low pH modifies thermal and thermo-chemical responses of mammalian cells. Int J Hyperthermia. 1986 Oct-Dec;2(4):379–387. doi: 10.3109/02656738609004968. [DOI] [PubMed] [Google Scholar]
- Hopkins N. K., Gorman R. R. Regulation of endothelial cell cyclic nucleotide metabolism by prostacyclin. J Clin Invest. 1981 Feb;67(2):540–546. doi: 10.1172/JCI110064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman M. R., Christensen K. L., Overgaard J. Hydralazine-induced enhancement of hyperthermic damage in a C3H mammary carcinoma in vivo. Int J Hyperthermia. 1989 Mar-Apr;5(2):123–136. doi: 10.3109/02656738909140442. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Jirtle R. L. Chemical modification of tumour blood flow. Int J Hyperthermia. 1988 Jul-Aug;4(4):355–371. doi: 10.3109/02656738809016490. [DOI] [PubMed] [Google Scholar]
- Lewis P. J., Dollery C. T. Clinical pharmacology and potential of prostacyclin. Br Med Bull. 1983 Jul;39(3):281–284. doi: 10.1093/oxfordjournals.bmb.a071834. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- O'Grady J., Warrington S., Moti M. J., Bunting S., Flower R., Fowle A. S., Higgs E. A., Moncada S. Effects of intravenous infusion of prostacyclin (PGI2) in man. Prostaglandins. 1980 Feb;19(2):319–332. doi: 10.1016/0090-6980(80)90030-1. [DOI] [PubMed] [Google Scholar]
- Okunieff P., Kallinowski F., Vaupel P., Neuringer L. J. Effects of hydralazine-induced vasodilation on the energy metabolism of murine tumors studied by in vivo 31P-nuclear magnetic resonance spectroscopy. J Natl Cancer Inst. 1988 Jul 20;80(10):745–750. doi: 10.1093/jnci/80.10.745. [DOI] [PubMed] [Google Scholar]
- Orchard M. A., Robinson C. Stability of prostacyclin in human plasma and whole blood: studies on the protective effect of albumin. Thromb Haemost. 1981 Oct;46(3):645–647. [PubMed] [Google Scholar]
- Overgaard J., Bichel P. The influence of hypoxia and acidity on the hyperthermic response of malignant cells in vitro. Radiology. 1977 May;123(2):511–514. doi: 10.1148/123.2.511. [DOI] [PubMed] [Google Scholar]
- Overgaard J., Nielsen O. S. The role of tissue environmental factors on the kinetics and morphology of tumor cells exposed to hyperthermia. Ann N Y Acad Sci. 1980;335:254–280. doi: 10.1111/j.1749-6632.1980.tb50753.x. [DOI] [PubMed] [Google Scholar]
- Prichard J. W., Alger J. R., Behar K. L., Petroff O. A., Shulman R. G. Cerebral metabolic studies in vivo by 31P NMR. Proc Natl Acad Sci U S A. 1983 May;80(9):2748–2751. doi: 10.1073/pnas.80.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd A. M., Ludden T. M., McNay J. L., Lin M. S. Hydralazine kinetics after single and repeated oral doses. Clin Pharmacol Ther. 1980 Dec;28(6):804–811. doi: 10.1038/clpt.1980.238. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Bhujwalla Z. M., Griffiths J. R., Maxwell R. J. Phosphorus-31 magnetic resonance spectroscopy and blood perfusion of the RIF-1 tumor following X-irradiation. Int J Radiat Oncol Biol Phys. 1989 Jan;16(1):155–164. doi: 10.1016/0360-3016(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Morris C. C. Blood flow and blood volume in a transplanted rat fibrosarcoma: comparison with various normal tissues. Radiother Oncol. 1990 Feb;17(2):153–165. doi: 10.1016/0167-8140(90)90103-4. [DOI] [PubMed] [Google Scholar]
- Vaupel P. Interrelationship between mean arterial blood pressure, blood flow, and vascular resistance in solid tumor tissue of DS-carcinosarcoma. Experientia. 1975 May 15;31(5):587–589. doi: 10.1007/BF01932474. [DOI] [PubMed] [Google Scholar]
- Voorhees W. D., 3rd, Babbs C. F. Hydralazine-enhanced selective heating of transmissible venereal tumor implants in dogs. Eur J Cancer Clin Oncol. 1982 Oct;18(10):1027–1033. doi: 10.1016/0277-5379(82)90252-8. [DOI] [PubMed] [Google Scholar]
- Wiig H., Tveit E., Hultborn R., Reed R. K., Weiss L. Interstitial fluid pressure in DMBA-induced rat mammary tumours. Scand J Clin Lab Invest. 1982 Apr;42(2):159–164. [PubMed] [Google Scholar]


