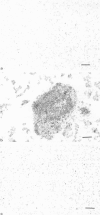
Abstract
Serine proteases, such as alpha-chymotrypsin or elastase, caused an aggregation of rat ascites tumour cell lines, AH-130, AH-109A and YS, in a protein free medium which preserved the cell viability. This aggregation, which was monitored spectrophotometrically, was dependent upon the protease activities and was resistant to treatment with either a calcium chelating reagent (EDTA) or neuraminidase. However, the tumour cell aggregates were redispersed by treatment with deoxyribonuclease I (DNase I). This dispersal effect was dependent upon the DNase activity. A possible relationship between the tumour cell aggregation and development of blood-borne metastasis was studied. An intravenous inoculation in rats of tumour cell aggregates performed by the alpha-chymotrypsin treatment resulted in significantly higher numbers of lung metastatic foci than an injection of single cells. When the re-separated single cells, prepared in vitro by treatment with DNase I following alpha-chymotrypsin treatment, were injected instead of the aggregates, the enhancement of metastasis was reversed. These enhancement and reversal effects were mimicked in vivo by intravenous injections of protease and nuclease following inoculation of a single cell suspension. That is, the number of metastatic foci caused by single cell inoculation followed by an intravenous alpha-chymotrypsin injection, was higher than that in a control group receiving PBS instead of alpha-chymotrypsin. Again, this augmentation was reversed by an injection of DNase I following alpha-chymotrypsin injection. Furthermore, an injection of DNase I alone itself reduced the starting number of metastases resulting from injection of the single tumour cell suspension. These data suggest that the metastatic behaviour of tumour cells may be increased by protease inducible DNA dependent cell aggregation should it occur in the blood stream.
Full text
PDF

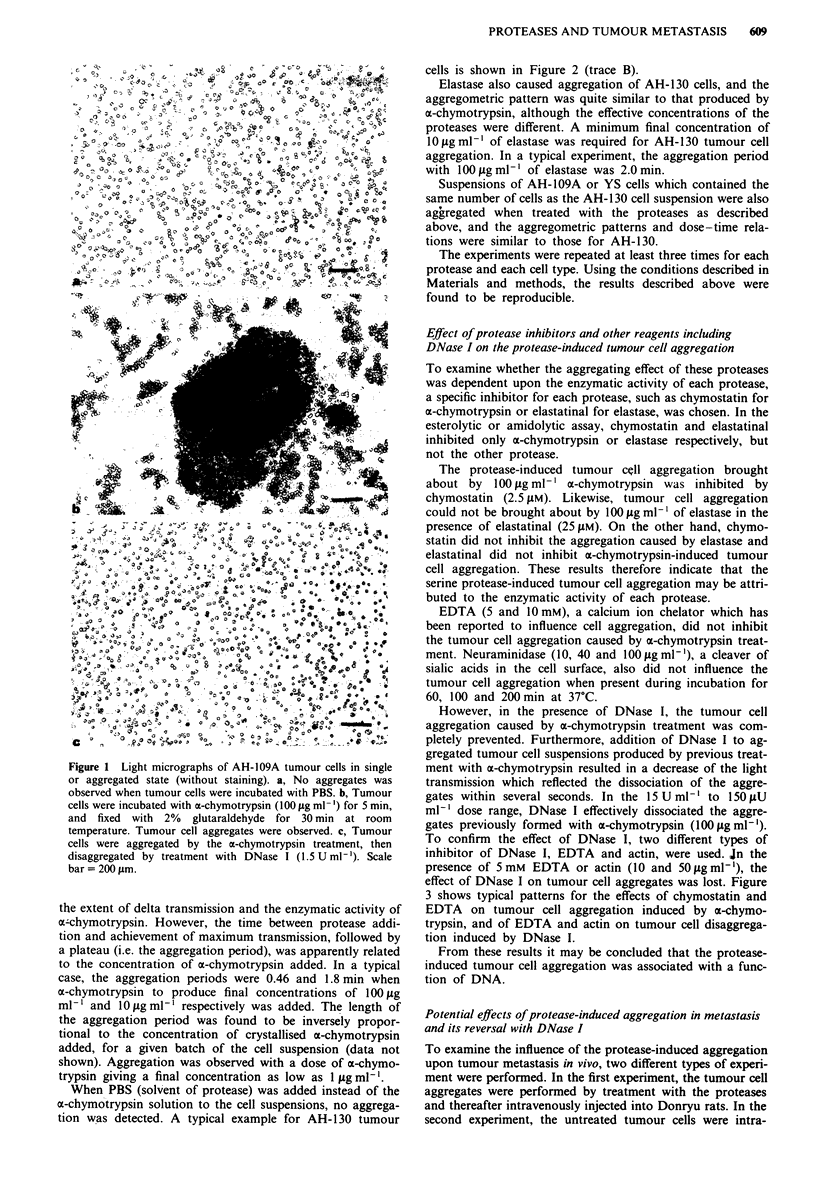
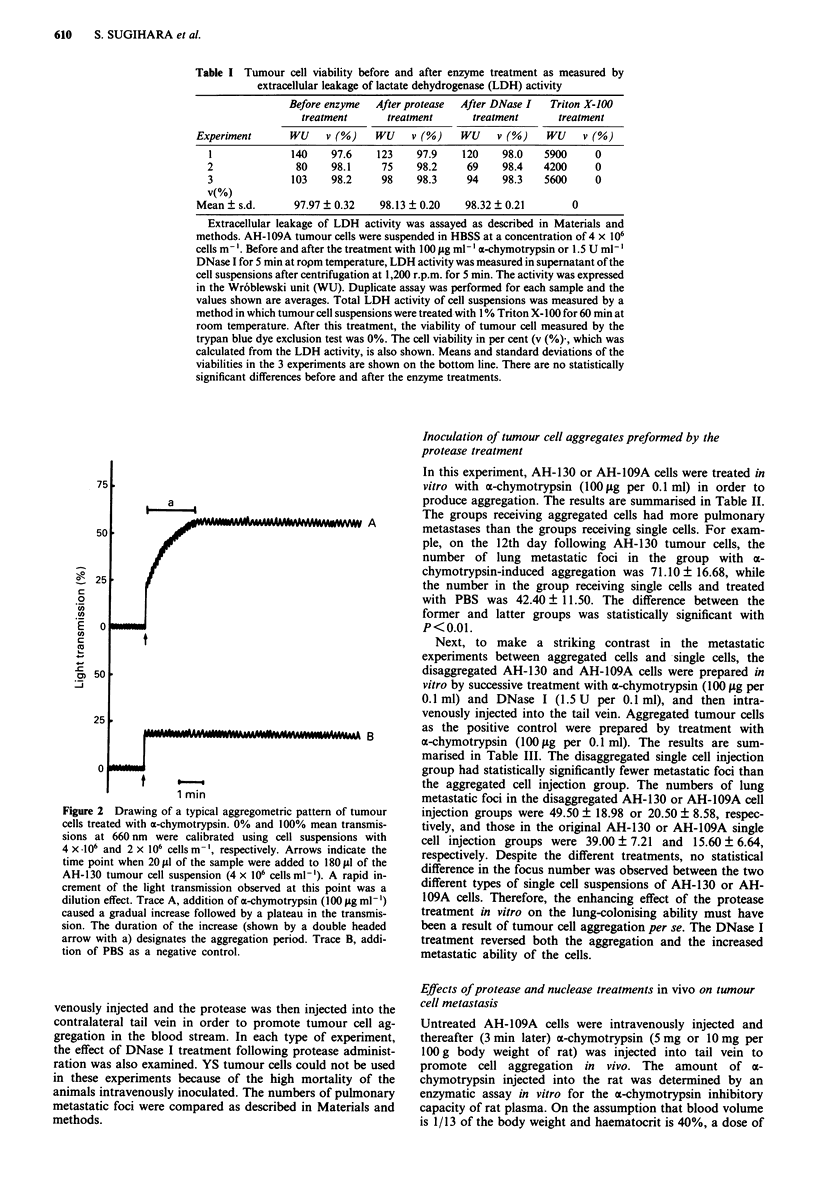
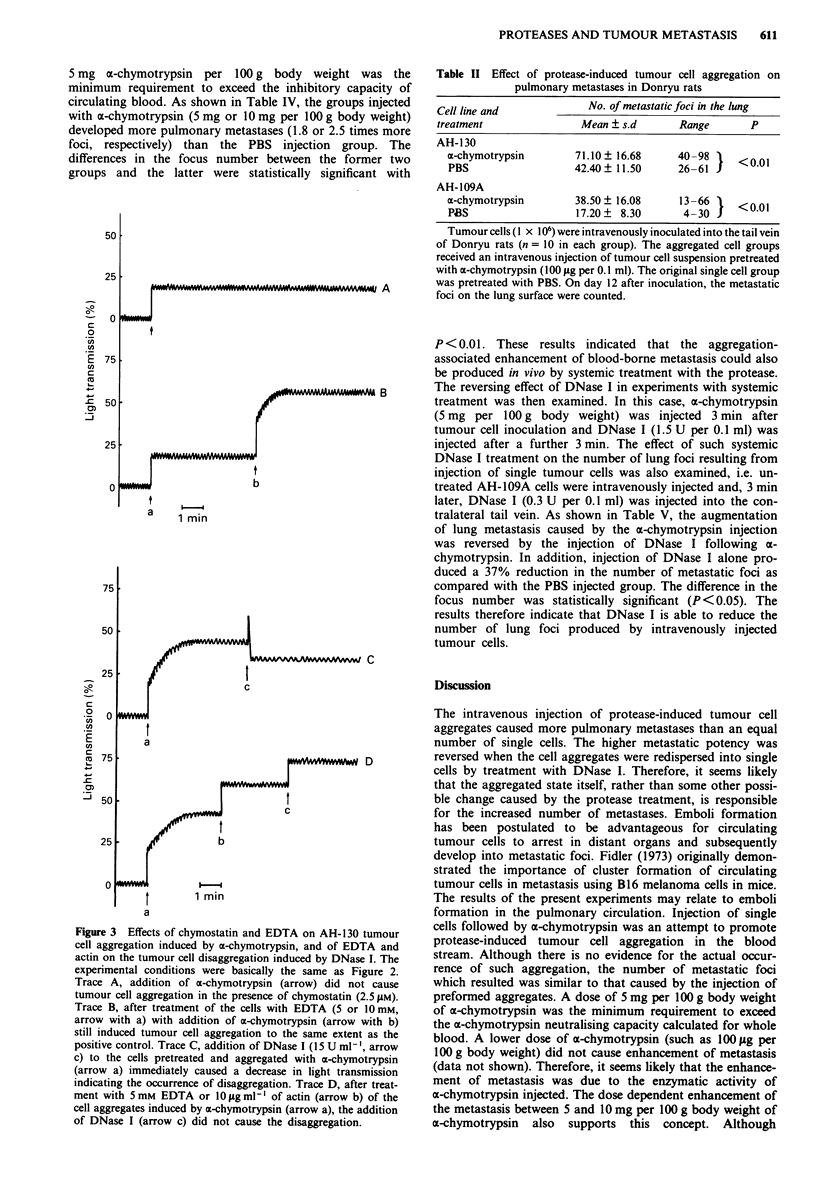


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUERBACH R., GROBSTEIN C. Inductive interaction of embryonic tissues after dissociation and reaggregation. Exp Cell Res. 1958 Oct;15(2):384–397. doi: 10.1016/0014-4827(58)90039-9. [DOI] [PubMed] [Google Scholar]
- Aggarwal S. K., Wagner R. W., McAllister P. K., Rosenberg B. Cell-surface-associated nucleic acid in tumorigenic cells made visible with platinum-pyrimidine complexes by electron microscopy. Proc Natl Acad Sci U S A. 1975 Mar;72(3):928–932. doi: 10.1073/pnas.72.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Bieber G. F., Brown A. E., Case K. R., Gersten D. M., Kimmerer T. W., Lione A. Biochemical parameters correlated with tumour cell implantation. Nature. 1973 Dec 21;246(5434):487–489. doi: 10.1038/246487a0. [DOI] [PubMed] [Google Scholar]
- CABAUD P. G., WROBLEWSKI F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Pathol. 1958 Sep;30(3):234–236. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fidler I. J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973 Mar;9(3):223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- Giraldi T., Sava G., Kopitar M., Brzin J., Turk V. Neutral proteinase inhibitors and antimetastatic effects in mice. Eur J Cancer. 1980 Apr;16(4):449–454. doi: 10.1016/0014-2964(80)90224-8. [DOI] [PubMed] [Google Scholar]
- Hara Y., Steiner M., Baldini M. G. Characterization of the platelet-aggregating activity of tumor cells. Cancer Res. 1980 Apr;40(4):1217–1222. [PubMed] [Google Scholar]
- Kinjo M. Lodgement and extravasation of tumour cells in blood-borne metastasis: an electron microscope study. Br J Cancer. 1978 Aug;38(2):293–301. doi: 10.1038/bjc.1978.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980 Mar 6;284(5751):67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Winkelhake J. L. Organ specificity of blood-borne tumour metastasis determined by cell adhesion? Nature. 1975 May 15;255(5505):230–232. doi: 10.1038/255230a0. [DOI] [PubMed] [Google Scholar]
- STEINBERG M. S. "ECM": its nature, origin and function in cell aggregation. Exp Cell Res. 1963 Apr;30:257–279. doi: 10.1016/0014-4827(63)90299-4. [DOI] [PubMed] [Google Scholar]
- Salganik R. I., Martynova R. P., Matienko N. A., Ronichevskaya G. M. Effect of deoxyribonuclease on the course of lymphatic leukaemia in AKR mice. Nature. 1967 Apr 1;214(5083):100–102. doi: 10.1038/214100a0. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Honn K. V., Sadler J. G., Turner W. A., Kimpson J. J., Taylor J. D. Cathepsin B activity in B16 melanoma cells: a possible marker for metastatic potential. Cancer Res. 1982 Mar;42(3):980–986. [PubMed] [Google Scholar]
- WEISS L. The effects of trypsin on the size, viability and dry mass of sarcoma 37 cells. Exp Cell Res. 1958 Feb;14(1):80–83. doi: 10.1016/0014-4827(58)90214-3. [DOI] [PubMed] [Google Scholar]
- Wang B. S., McLoughlin G. A., Richie J. P., Mannick J. A. Correlation of the production of plasminogen activator with tumor metastasis in B16 mouse melanoma cell lines. Cancer Res. 1980 Feb;40(2):288–292. [PubMed] [Google Scholar]
- Wexler H. Accurate identification of experimental pulmonary metastases. J Natl Cancer Inst. 1966 Apr;36(4):641–645. doi: 10.1093/jnci/36.4.641. [DOI] [PubMed] [Google Scholar]