Abstract
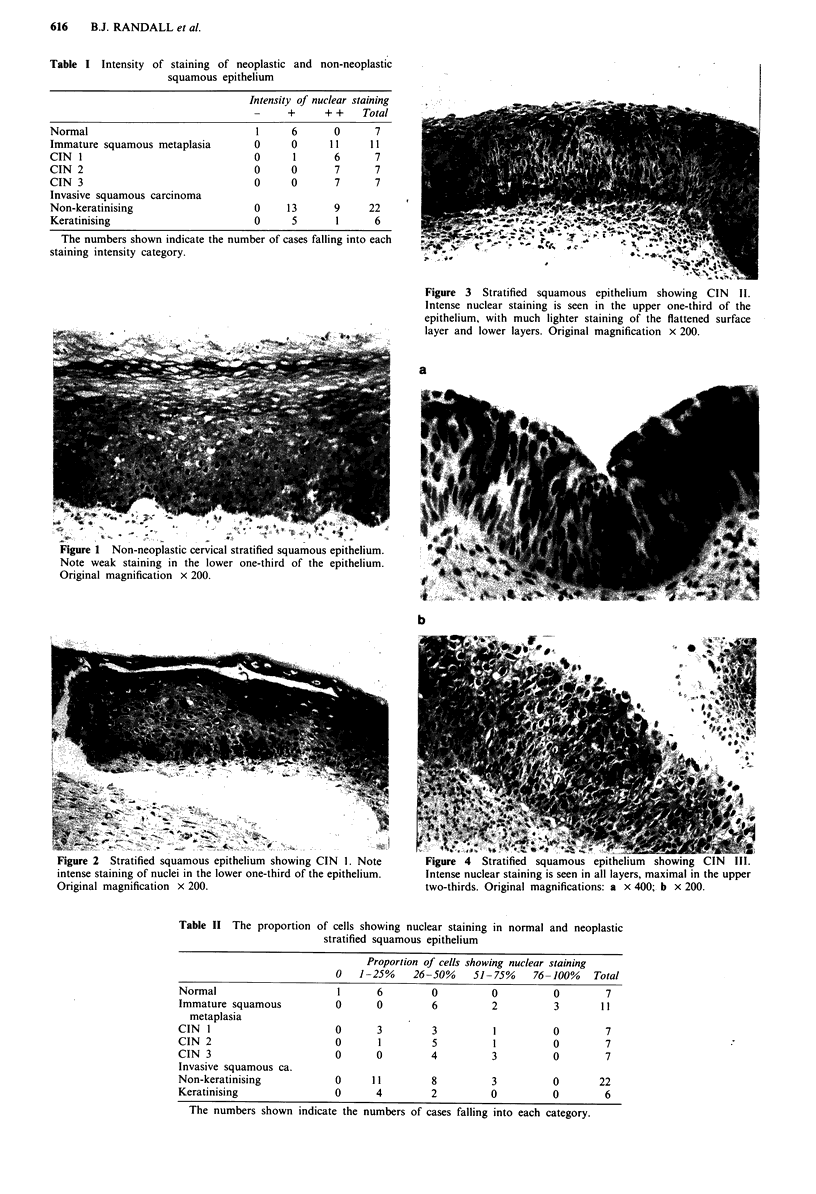
Using an indirect immunohistochemical technique on paraffin sections, employing a polyclonal antibody to the acidic (placental) form of glutathione-S-transferase (GST), we have evaluated cytoplasmic and nuclear staining in a series of 67 cervical biopsies including normal non neoplastic tissue, immature squamous metaplasia, all grades of cervical intraepithelial neoplasia (CIN) and invasive carcinomas of keratinising and non-keratinising types. No differences in cytoplasmic staining between the varied lesions studied were seen. However, there were marked differences in nuclear staining. While normal non-neoplastic stratified squamous epithelium showed weak staining of the lower one-third of the epithelium only, in immature squamous metaplasia and in all grades of CIN there was intense nuclear staining in all layers of the epithelium. Invasive carcinomas showed generally less intense nuclear staining than CIN lesions. Endocervical cell nuclei also showed intense nuclear staining. These findings indicate that GST is of limited use as a marker of transformation in the human cervix uteri.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus B., Kiberu S., Purvis J., Wilkinson L., Horne C. H. Cytokeratins in cervical dysplasia and neoplasia: a comparative study of immunohistochemical staining using monoclonal antibodies NCL-5D3, CAM 5.2, and PKK1. J Pathol. 1988 May;155(1):71–75. doi: 10.1002/path.1711550111. [DOI] [PubMed] [Google Scholar]
- Angus B., Purvis J., Stock D., Westley B. R., Samson A. C., Routledge E. G., Carpenter F. H., Horne C. H. NCL-5D3: a new monoclonal antibody recognizing low molecular weight cytokeratins effective for immunohistochemistry using fixed paraffin-embedded tissue. J Pathol. 1987 Dec;153(4):377–384. doi: 10.1002/path.1711530411. [DOI] [PubMed] [Google Scholar]
- Bobrow L. G., Makin C. A., Law S., Bodmer W. F. Expression of low molecular weight cytokeratin proteins in cervical neoplasia. J Pathol. 1986 Feb;148(2):135–140. doi: 10.1002/path.1711480203. [DOI] [PubMed] [Google Scholar]
- Brescia R. J., Jenson A. B., Lancaster W. D., Kurman R. J. The role of human papillomaviruses in the pathogenesis and histologic classification of precancerous lesions of the cervix. Hum Pathol. 1986 Jun;17(6):552–559. doi: 10.1016/s0046-8177(86)80126-5. [DOI] [PubMed] [Google Scholar]
- Burns J., Graham A. K., Frank C., Fleming K. A., Evans M. F., McGee J. O. Detection of low copy human papilloma virus DNA and mRNA in routine paraffin sections of cervix by non-isotopic in situ hybridisation. J Clin Pathol. 1987 Aug;40(8):858–864. doi: 10.1136/jcp.40.8.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M., Freeth M., Crocker J. Intraepithelial neoplasia, human papilloma virus infection and argyrophilic nucleoprotein in cervical epithelium. Histopathology. 1988 Nov;13(5):561–567. doi: 10.1111/j.1365-2559.1988.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Elliott P. M., Tattersall M. H., Coppleson M., Russell P., Wong F., Coates A. S., Solomon H. J., Bannatyne P. M., Atkinson K. H., Murray J. C. Changing character of cervical cancer in young women. BMJ. 1989 Feb 4;298(6669):288–290. doi: 10.1136/bmj.298.6669.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray R. E., Husain O. A., To A. C., Watts K. C., Lader S., Rogers G. T., Taylor-Papadimitriou J., Morris N. F. The value of immunohistochemical markers in the diagnosis of cervical neoplasia. Br J Obstet Gynaecol. 1984 Oct;91(10):1037–1041. doi: 10.1111/j.1471-0528.1984.tb03684.x. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Laisney V., Nguyen Van Cong, Gross M. S., Frezal J. Human genes for glutathione S-transferases. Hum Genet. 1984;68(3):221–227. doi: 10.1007/BF00418392. [DOI] [PubMed] [Google Scholar]
- Ludwig M. E., Lowell D. M., Livolsi V. A. Cervical condylomatous atypia and its relationship to cervical neoplasia. Am J Clin Pathol. 1981 Sep;76(3):255–262. doi: 10.1093/ajcp/76.3.255. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- McCance D. J. Human papillomaviruses and cancer. Biochim Biophys Acta. 1986;823(3):195–205. doi: 10.1016/0304-419x(86)90002-8. [DOI] [PubMed] [Google Scholar]
- Millett J. A., Husain O. A., Bitensky L., Chayen J. Feulgen-hydrolysis profiles in cells exfoliated from the cervix uteri: a potential aid in the diagnosis of malignancy. J Clin Pathol. 1982 Mar;35(3):345–349. doi: 10.1136/jcp.35.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. B., Gatter K. C., Pulford K., Haynes P., Charnock M., Taylor-Papadimitriou J., Lane E. B., Mason D. Y. Cervical wart virus infection, intraepithelial neoplasia and carcinoma; an immunohistological study using a panel of monoclonal antibodies. Br J Obstet Gynaecol. 1983 Nov;90(11):1069–1081. doi: 10.1111/j.1471-0528.1983.tb06447.x. [DOI] [PubMed] [Google Scholar]
- Raju G. C. Expression of the cytokeratin marker CAM 5.2 in cervical neoplasia. Histopathology. 1988 Apr;12(4):437–443. doi: 10.1111/j.1365-2559.1988.tb01958.x. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Lewis A. D., Wolf C. R., Hayes J. D., Hall A., Proctor S. J., Harris A. L., Hickson I. D. Reduced levels of drug-induced DNA cross-linking in nitrogen mustard-resistant Chinese hamster ovary cells expressing elevated glutathione S-transferase activity. Cancer Res. 1987 Nov 15;47(22):6022–6027. [PubMed] [Google Scholar]
- Seidegård J., Pero R. W., Miller D. G., Beattie E. J. A glutathione transferase in human leukocytes as a marker for the susceptibility to lung cancer. Carcinogenesis. 1986 May;7(5):751–753. doi: 10.1093/carcin/7.5.751. [DOI] [PubMed] [Google Scholar]
- Shiratori Y., Soma Y., Maruyama H., Sato S., Takano A., Sato K. Immunohistochemical detection of the placental form of glutathione S-transferase in dysplastic and neoplastic human uterine cervix lesions. Cancer Res. 1987 Dec 15;47(24 Pt 1):6806–6809. [PubMed] [Google Scholar]
- Sincock A. M., Middleton J., Moncrieff D. Towards an automated procedure for the quantitative cytological screening of cervical neoplasms. J Clin Pathol. 1983 May;36(5):535–538. doi: 10.1136/jcp.36.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandidos D. A., Anderson M. L. Oncogenes and onco-suppressor genes: their involvement in cancer. J Pathol. 1989 Jan;157(1):1–10. doi: 10.1002/path.1711570102. [DOI] [PubMed] [Google Scholar]
- Spriggs A. I., Boddington M. M. Progression and regression of cervical lesions. Review of smears from women followed without initial biopsy or treatment. J Clin Pathol. 1980 Jun;33(6):517–522. doi: 10.1136/jcp.33.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterrett G. F., Alessandri L. M., Pixley E., Kulski J. K. Assessment of precancerous lesions of the uterine cervix for evidence of human papillomavirus infection: a histological and immunohistochemical study. Pathology. 1987 Jan;19(1):84–90. doi: 10.3109/00313028709065144. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]
- Whittaker J. R., Samy A. M., Sunter J. P., Sinha D. P., Monaghan J. M. Cytokeratin expression in cervical epithelium: an immunohistological study of normal, wart virus-infected and neoplastic tissue. Histopathology. 1989 Feb;14(2):151–160. doi: 10.1111/j.1365-2559.1989.tb02125.x. [DOI] [PubMed] [Google Scholar]