Abstract
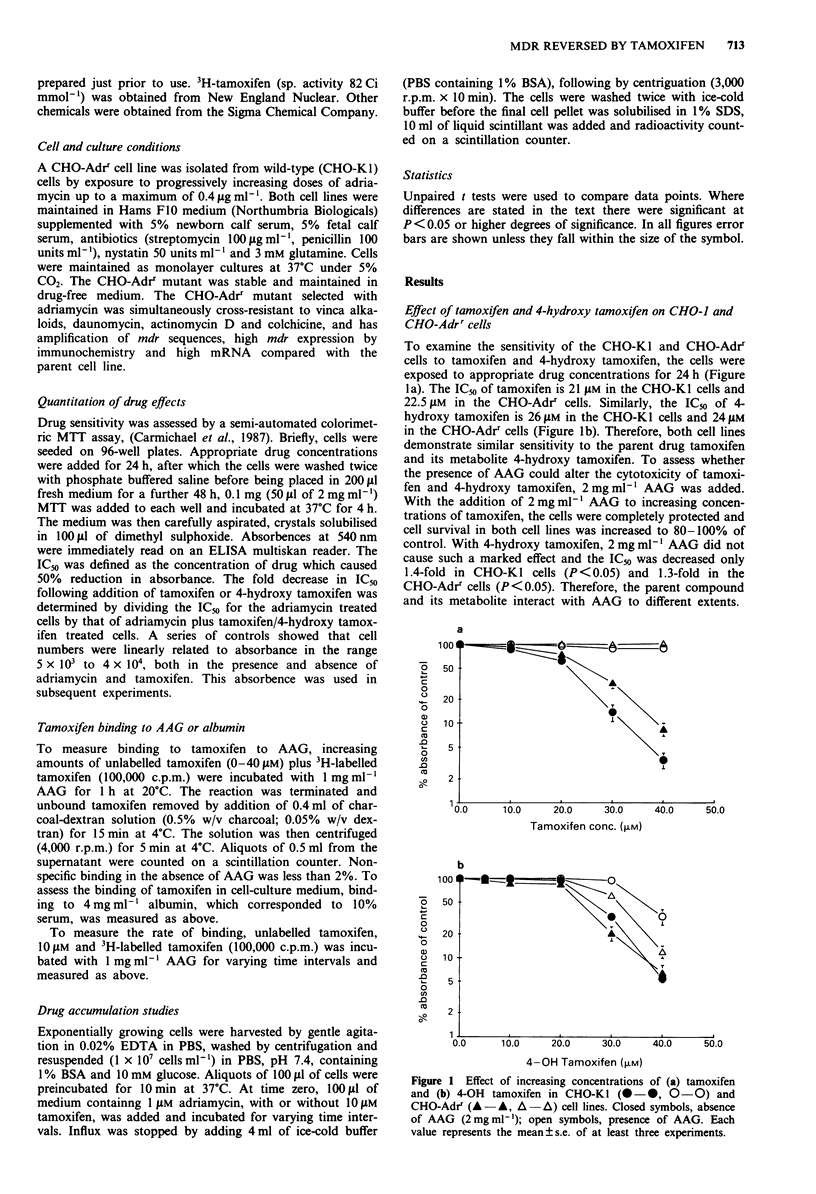
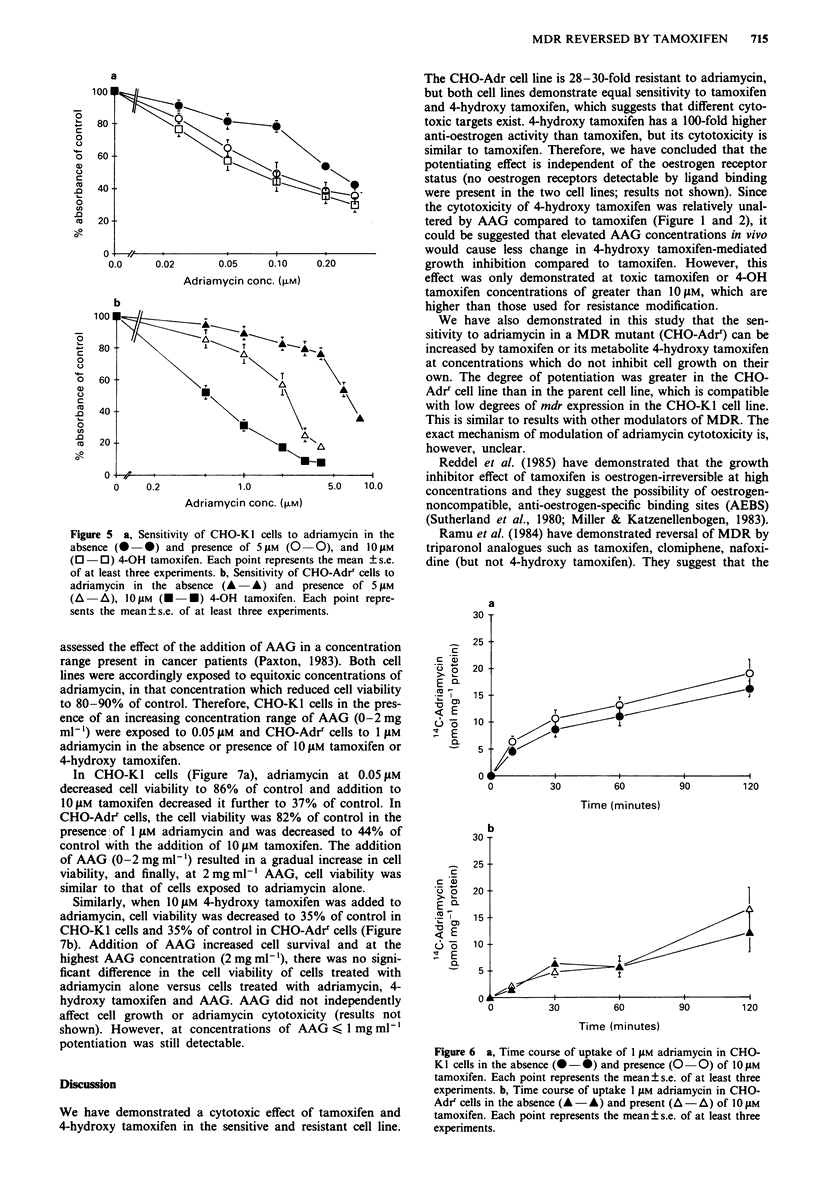
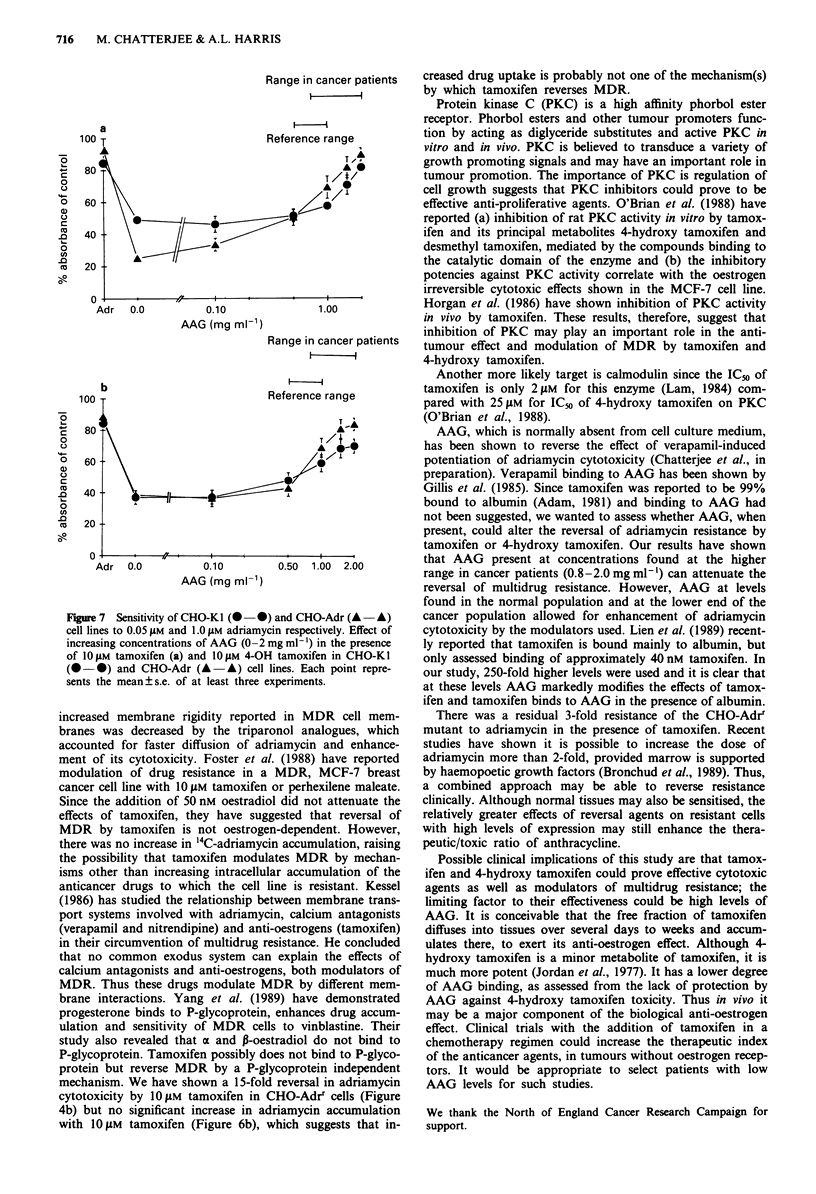
Tamoxifen and 4-OH tamoxifen were used to reverse multidrug resistance (MDR) in CHO cells with acquired resistance to adriamycin (CHO-Adrr). Because alpha 1 acid glycoprotein (AAG) can bind a range of calcium channel blockers that also reverse MDR and rises in malignancy, its interactions with tamoxifen and 4-OH tamoxifen were also studied. Tamoxifen decreased the IC50 of 10 microM adriamycin 4.8-fold in the parent CHO-K1 cell line and 16-fold in CHO-Adrr. Similarly 4-OH tamoxifen decreased the IC50 3-fold in the parent cells, but 13-fold in the resistant cells. Tamoxifen and 4-OH tamoxifen were similarly potent in reversing MDR, although their anti-oestrogen potency differs 100-fold. AAG was added in increasing concentrations to the combination of adriamycin and tamoxifen. As AAG concentrations increased from 0.5 to 2 mg ml-1 (the range found in vivo) the effect of tamoxifen on reversing MDR was gradually decreased. At the highest AAG concentrations, there was complete reversal of the effects of both tamoxifen and 4-OH tamoxifen. AAG was found to bind 3H-tamoxifen in a non-saturable non-specific manner, in contrast to the binding of tamoxifen to albumin. Thus the use of tamoxifen as a reversal agent for MDR in vivo may be impaired by high binding to AAG. However, at the lower range of normal values of AAG, there was still an effect of 10 microM tamoxifen. It may be desirable to select patients for modifier studies based on AAG plasma levels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson A. B., 3rd, Trump D. L., Koeller J. M., Egorin M. I., Olman E. A., Witte R. S., Davis T. E., Tormey D. C. Phase I study of vinblastine and verapamil given by concurrent iv infusion. Cancer Treat Rep. 1985 Jul-Aug;69(7-8):795–799. [PubMed] [Google Scholar]
- Biedler J. L., Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Res. 1970 Apr;30(4):1174–1184. [PubMed] [Google Scholar]
- Bronchud M. H., Howell A., Crowther D., Hopwood P., Souza L., Dexter T. M. The use of granulocyte colony-stimulating factor to increase the intensity of treatment with doxorubicin in patients with advanced breast and ovarian cancer. Br J Cancer. 1989 Jul;60(1):121–125. doi: 10.1038/bjc.1989.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Chauffert B., Martin M., Hammann A., Michel M. F., Martin F. Amiodarone-induced enhancement of doxorubicin and 4'-deoxydoxorubicin cytotoxicity to rat colon cancer cells in vitro and in vivo. Cancer Res. 1986 Feb;46(2):825–830. [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Foster B. J., Grotzinger K. R., McKoy W. M., Rubinstein L. V., Hamilton T. C. Modulation of induced resistance to adriamycin in two human breast cancer cell lines with tamoxifen or perhexiline maleate. Cancer Chemother Pharmacol. 1988;22(2):147–152. doi: 10.1007/BF00257313. [DOI] [PubMed] [Google Scholar]
- Gillis A. M., Yee Y. G., Kates R. E. Binding of antiarrhythmic drugs to purified human alpha 1-acid glycoprotein. Biochem Pharmacol. 1985 Dec 15;34(24):4279–4282. doi: 10.1016/0006-2952(85)90285-0. [DOI] [PubMed] [Google Scholar]
- Gros P., Croop J., Roninson I., Varshavsky A., Housman D. E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):337–341. doi: 10.1073/pnas.83.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan K., Cooke E., Hallett M. B., Mansel R. E. Inhibition of protein kinase C mediated signal transduction by tamoxifen. Importance for antitumour activity. Biochem Pharmacol. 1986 Dec 15;35(24):4463–4465. doi: 10.1016/0006-2952(86)90764-1. [DOI] [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Jordan V. C., Collins M. M., Rowsby L., Prestwich G. A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol. 1977 Nov;75(2):305–316. doi: 10.1677/joe.0.0750305. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Kessel D. Interactions among membrane transport systems: anthracyclines, calcium antagonists and anti-estrogens. Biochem Pharmacol. 1986 Aug 15;35(16):2825–2826. doi: 10.1016/0006-2952(86)90196-6. [DOI] [PubMed] [Google Scholar]
- Lam H. Y. Tamoxifen is a calmodulin antagonist in the activation of cAMP phosphodiesterase. Biochem Biophys Res Commun. 1984 Jan 13;118(1):27–32. doi: 10.1016/0006-291x(84)91062-3. [DOI] [PubMed] [Google Scholar]
- Lien E. A., Solheim E., Lea O. A., Lundgren S., Kvinnsland S., Ueland P. M. Distribution of 4-hydroxy-N-desmethyltamoxifen and other tamoxifen metabolites in human biological fluids during tamoxifen treatment. Cancer Res. 1989 Apr 15;49(8):2175–2183. [PubMed] [Google Scholar]
- Miller M. A., Katzenellenbogen B. S. Characterization and quantitation of antiestrogen binding sites in estrogen receptor-positive and -negative human breast cancer cell lines. Cancer Res. 1983 Jul;43(7):3094–3100. [PubMed] [Google Scholar]
- O'Brian C. A., Ward N. E., Anderson B. W. Role of specific interactions between protein kinase C and triphenylethylenes in inhibition of the enzyme. J Natl Cancer Inst. 1988 Dec 21;80(20):1628–1633. doi: 10.1093/jnci/80.20.1628. [DOI] [PubMed] [Google Scholar]
- Paxton J. W. Alpha 1 -acid glycoprotein and binding of basic drugs. Methods Find Exp Clin Pharmacol. 1983 Nov;5(9):635–648. [PubMed] [Google Scholar]
- Ramu A., Fuks Z., Gatt S., Glaubiger D. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by perhexiline maleate. Cancer Res. 1984 Jan;44(1):144–148. [PubMed] [Google Scholar]
- Ramu A., Glaubiger D., Fuks Z. Reversal of acquired resistance to doxorubicin in P388 murine leukemia cells by tamoxifen and other triparanol analogues. Cancer Res. 1984 Oct;44(10):4392–4395. [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Hall R. E., Sutherland R. L. Differential sensitivity of human breast cancer cell lines to the growth-inhibitory effects of tamoxifen. Cancer Res. 1985 Apr;45(4):1525–1531. [PubMed] [Google Scholar]
- Robertson D. W., Katzenellenbogen J. A., Long D. J., Rorke E. A., Katzenellenbogen B. S. Tamoxifen antiestrogens. A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Steroid Biochem. 1982 Jan;16(1):1–13. doi: 10.1016/0022-4731(82)90137-6. [DOI] [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Sewell J. L., Meyers M. B., Biedler J. L., Felsted R. L. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J Biol Chem. 1987 Jun 5;262(16):7884–7888. [PubMed] [Google Scholar]
- Sutherland R. L., Murphy L. C., San Foo M., Green M. D., Whybourne A. M., Krozowski Z. S. High-affinity anti-oestrogen binding site distinct from the oestrogen receptor. Nature. 1980 Nov 20;288(5788):273–275. doi: 10.1038/288273a0. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Yang C. P., DePinho S. G., Greenberger L. M., Arceci R. J., Horwitz S. B. Progesterone interacts with P-glycoprotein in multidrug-resistant cells and in the endometrium of gravid uterus. J Biol Chem. 1989 Jan 15;264(2):782–788. [PubMed] [Google Scholar]


