Abstract
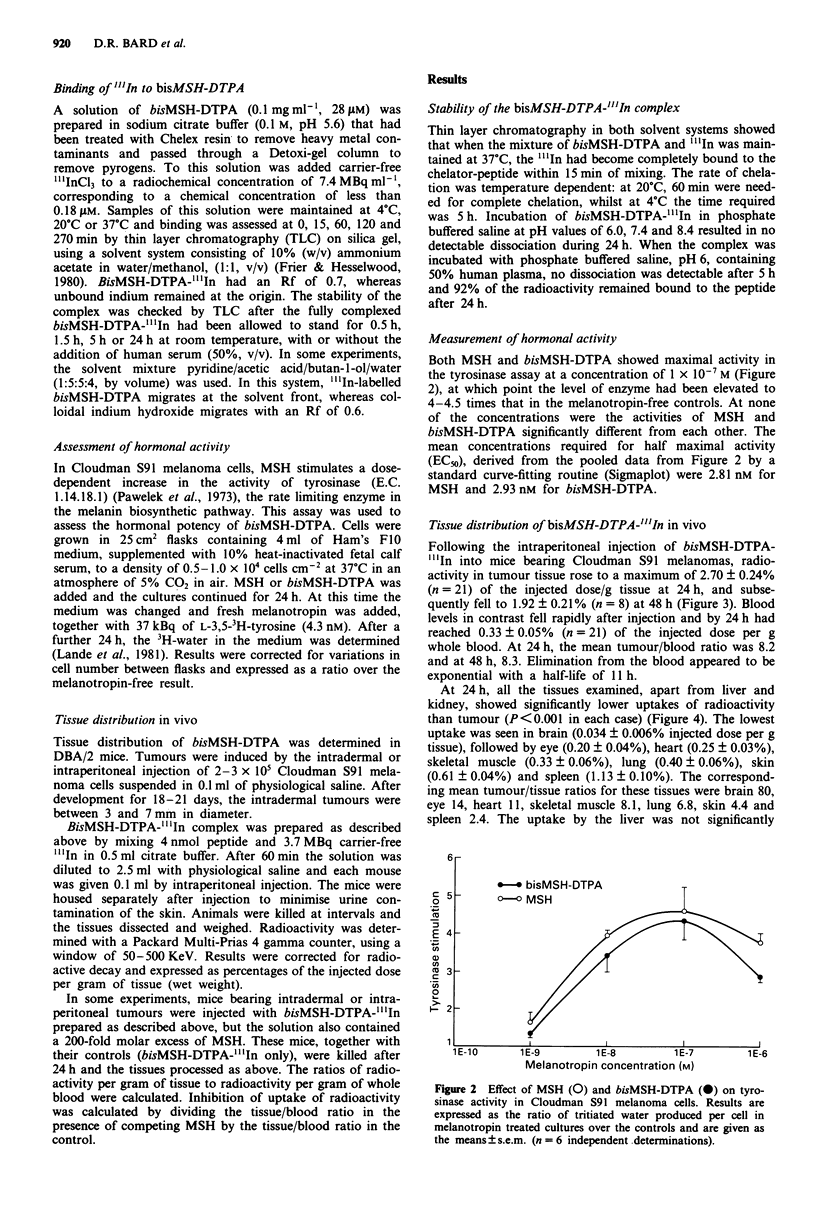
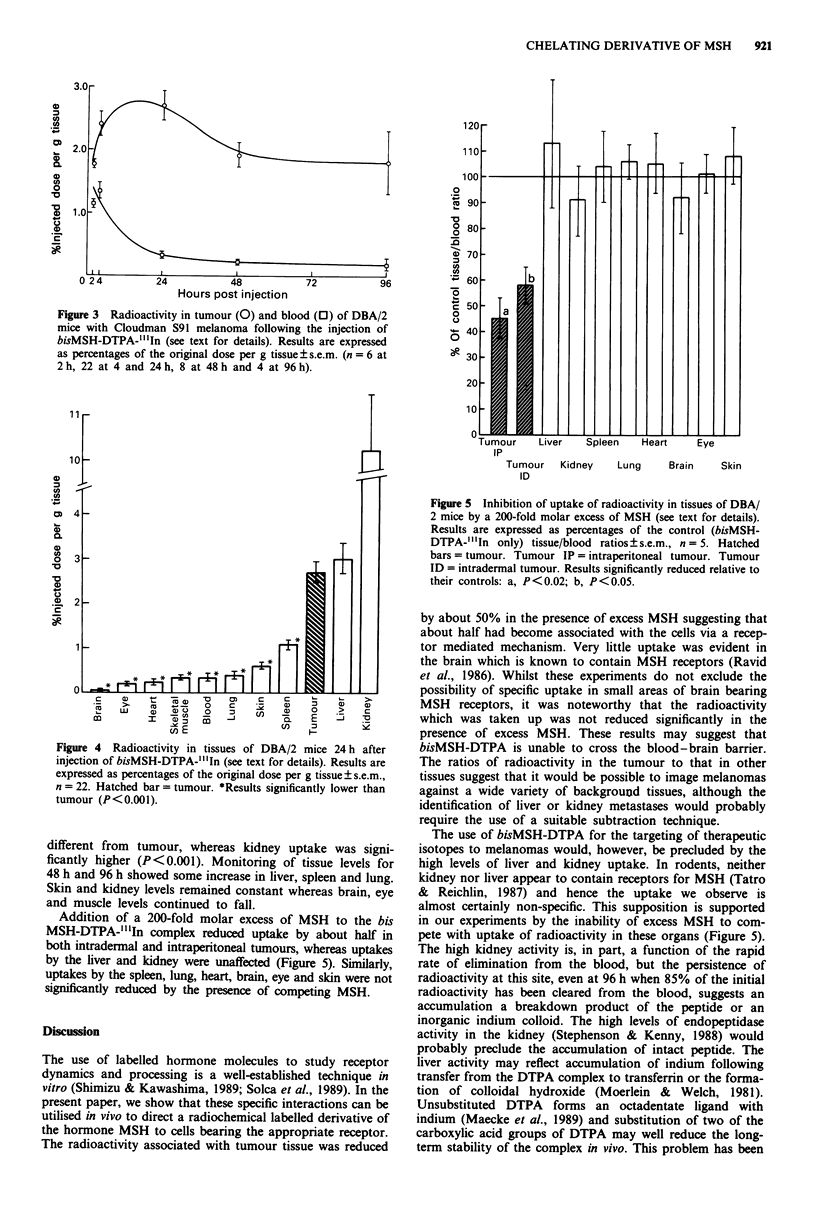
A chelating derivative of alpha-melanocyte stimulating hormone (MSH) has been synthesised, in which two molecules of the hormone are cross-linked by diethylenetriamine pentaacetic acid (DTPA). This compound, bisMSH-DTPA, was equipotent with MSH in an in vitro tyrosinase assay with Cloudman S91 melanoma cells. When DBA/2 mice bearing the same tumour were injected with bisMSH-DTPA labelled with the gamma-emitting isotope indium-111 (111In), the radioactivity became rapidly associated with the melanoma tissue. By 24 h post-injection, radioactivity in tumour tissue was significantly higher (P less than 0.001) than in spleen, lung, brain, eye and skin. Uptake of radioactivity by the tumours was inhibited by a 200-fold molar excess of MSH, whereas uptake by liver, kidney, spleen, lung, brain, eye and skin was unaffected. We conclude that bisMSH-DTPA may offer an alternative to antibody targeting in the imaging of malignant melanoma.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castrucci A. M., Hadley M. E., Sawyer T. K., Hruby V. J. Enzymological studies of melanotropins. Comp Biochem Physiol B. 1984;78(3):519–524. doi: 10.1016/0305-0491(84)90090-7. [DOI] [PubMed] [Google Scholar]
- Chaturvedi D. N., Hruby V. J., Castrucci A. M., Kreutzfeld K. L., Hadley M. E. Synthesis and biological evaluation of the superagonist [N alpha-chlorotriazinylaminofluorescein-Ser1,Nle4,D-Phe7]-al pha-MSH. J Pharm Sci. 1985 Mar;74(3):237–240. doi: 10.1002/jps.2600740303. [DOI] [PubMed] [Google Scholar]
- Chaturvedi D. N., Knittel J. J., Hruby V. J., Castrucci A. M., Hadley M. E. Synthesis and biological actions of highly potent and prolonged acting biotin-labeled melanotropins. J Med Chem. 1984 Nov;27(11):1406–1410. doi: 10.1021/jm00377a005. [DOI] [PubMed] [Google Scholar]
- De Wied D., Jolles J. Neuropeptides derived from pro-opiocortin: behavioral, physiological, and neurochemical effects. Physiol Rev. 1982 Jul;62(3):976–1059. doi: 10.1152/physrev.1982.62.3.976. [DOI] [PubMed] [Google Scholar]
- Ghanem G. E., Comunale G., Libert A., Vercammen-Grandjean A., Lejeune F. J. Evidence for alpha-melanocyte-stimulating hormone (alpha-MSH) receptors on human malignant melanoma cells. Int J Cancer. 1988 Feb 15;41(2):248–255. doi: 10.1002/ijc.2910410216. [DOI] [PubMed] [Google Scholar]
- LERNER A. B., MCGUIRE J. S. Effect of alpha- and betamelanocyte stimulating hormones on the skin colour of man. Nature. 1961 Jan 21;189:176–179. doi: 10.1038/189176a0. [DOI] [PubMed] [Google Scholar]
- Lande S., Pawelek J., Lerner A. B., Emanuel J. R. Assay of melanotropic peptides in an in vitro mammalian system. J Invest Dermatol. 1981 Aug;77(2):244–245. doi: 10.1111/1523-1747.ep12480145. [DOI] [PubMed] [Google Scholar]
- Larson S. M., Brown J. P., Wright P. W., Carrasquillo J. A., Hellström I., Hellström K. E. Imaging of melanoma with L-131-labeled monoclonal antibodies. J Nucl Med. 1983 Feb;24(2):123–129. [PubMed] [Google Scholar]
- Liu M. A., Nussbaum S. R., Eisen H. N. Hormone conjugated with antibody to CD3 mediates cytotoxic T cell lysis of human melanoma cells. Science. 1988 Jan 22;239(4838):395–398. doi: 10.1126/science.3257303. [DOI] [PubMed] [Google Scholar]
- Maecke H. R., Riesen A., Ritter W. The molecular structure of indium-DTPA. J Nucl Med. 1989 Jul;30(7):1235–1239. [PubMed] [Google Scholar]
- Moerlein S. M., Welch M. J. The chemistry of gallium and indium as related to radiopharmaceutical production. Int J Nucl Med Biol. 1981;8(4):277–287. doi: 10.1016/0047-0740(81)90034-6. [DOI] [PubMed] [Google Scholar]
- Pawelek J., Wong G., Sansone M., Morowitz J. Molecular biology of pigment cells. Molecular controls in mammalian pigmentation. Yale J Biol Med. 1973 Dec;46(5):430–443. [PMC free article] [PubMed] [Google Scholar]
- Ravid R., Swaab D. F., Van der Woude T. P., Boer G. J. Immunocytochemically stained binding sites for oxytocin and alpha-melanocyte-stimulating hormone in rat brain following ventricular administration. Brain Res. 1986 Aug 6;379(2):404–408. doi: 10.1016/0006-8993(86)90800-0. [DOI] [PubMed] [Google Scholar]
- Reckel R. Monoclonal antibodies: clinical applications. Adv Clin Chem. 1989;27:355–415. doi: 10.1016/s0065-2423(08)60187-0. [DOI] [PubMed] [Google Scholar]
- Sawyer T. K., Sanfilippo P. J., Hruby V. J., Engel M. H., Heward C. B., Burnett J. B., Hadley M. E. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Beatty S. M., Oldham R. K., Morgan A. C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985 Feb;45(2):879–885. [PubMed] [Google Scholar]
- Shimizu A., Kawashima S. Kinetic study of internalization and degradation of 131I-labeled follicle-stimulating hormone in mouse Sertoli cells and its relevance to other systems. J Biol Chem. 1989 Aug 15;264(23):13632–13638. [PubMed] [Google Scholar]
- Siegrist W., Oestreicher M., Stutz S., Girard J., Eberle A. N. Radioreceptor assay for alpha-MSH using mouse B16 melanoma cells+. J Recept Res. 1988;8(1-4):323–343. doi: 10.3109/10799898809048996. [DOI] [PubMed] [Google Scholar]
- Solca F., Siegrist W., Drozdz R., Girard J., Eberle A. N. The receptor for alpha-melanotropin of mouse and human melanoma cells. Application of a potent alpha-melanotropin photoaffinity label. J Biol Chem. 1989 Aug 25;264(24):14277–14281. [PubMed] [Google Scholar]
- Stephenson S. L., Kenny A. J. The metabolism of neuropeptides. Hydrolysis of peptides by the phosphoramidon-insensitive rat kidney enzyme 'endopeptidase-2' and by rat microvillar membranes. Biochem J. 1988 Oct 1;255(1):45–51. doi: 10.1042/bj2550045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatro J. B., Atkins M., Mier J. W., Hardarson S., Wolfe H., Smith T., Entwistle M. L., Reichlin S. Melanotropin receptors demonstrated in situ in human melanoma. J Clin Invest. 1990 Jun;85(6):1825–1832. doi: 10.1172/JCI114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatro J. B., Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987 Nov;121(5):1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- Wilson J. F., Morgan M. A. Plasma concentrations of alpha-melanotropin in the rat during the acquisition and extinction of conditioned avoidance behaviour and during the acquisition of maze learning behaviour. Psychopharmacology (Berl) 1980;68(1):67–72. doi: 10.1007/BF00426652. [DOI] [PubMed] [Google Scholar]


