Abstract
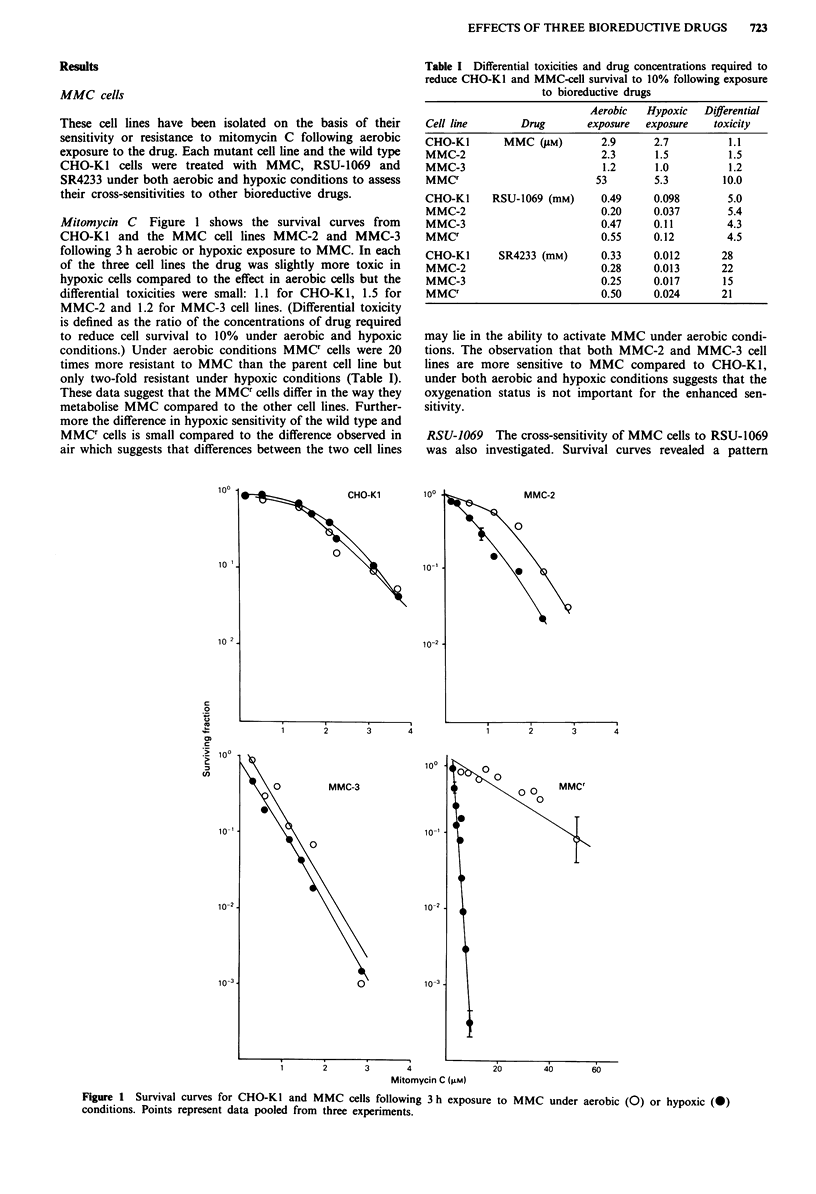
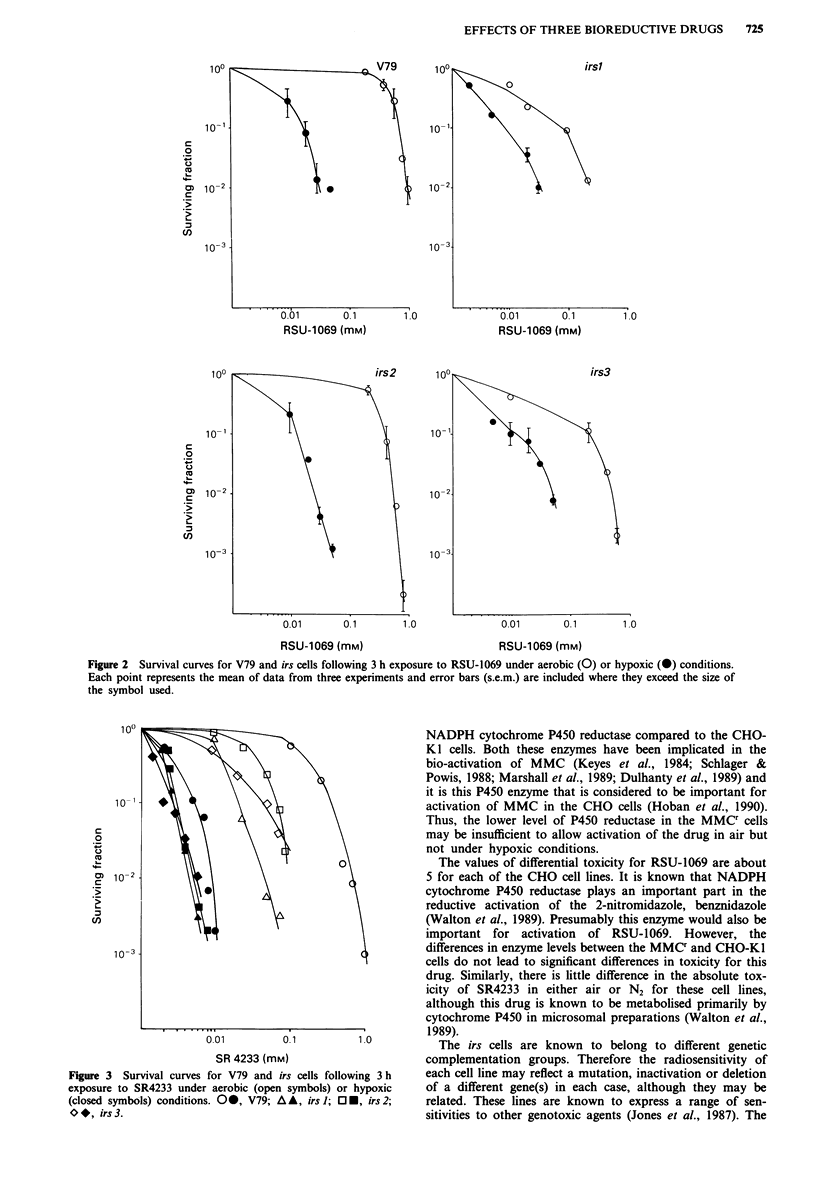
We have investigated the cross-sensitivity of a number of cell lines to three different classes of bioreductive drugs under both aerobic and hypoxic conditions. The cell lines used were selected for their sensitivity to DNA-damaging agents and fall into two groups. One group, MMC cells derived from CHO-K1 cells (Robson et al., 1985), show a range of sensitivities to mitomycin C in air. The second group, irs cells were cloned from V79 Chinese hamster fibroblasts (Jones et al., 1987) and exhibit sensitivity to ionising radiation. The sensitivity of both groups of cells to mitomycin C (MMC), RSU-1069 and SR4233 was assessed under aerobic and hypoxic conditions. No difference in aerobic or hypoxic toxicity of MMC was observed for CHO-K1 or MMC sensitive cell lines (MMC-2 and MMC-3). However, the MMC-resistant cell line (MMCr) was 10 times more sensitive under hypoxic than aerobic conditions. This suggests that MMCr cells lack or are deficient in the enzymes responsible for activating MMC under aerobic conditions compared to other MMC cells. In contrast, differential toxicities of between 3 and 30 have been observed for all CHO cells treated with RSU-1069 and SR4233. Treatment of V79 and irs cells with RSU-1069 and SR4233 also resulted in selective toxicity towards hypoxic cells. Differential toxicities between 50 and 100 were observed for V79 cells. For both RSU-1069 and SR4233, the hypoxic toxicities were similar in V79 and irs cells but in air, the radiation sensitive cells were up to 10 times more sensitive than wild type cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Ahmed I., Sheldon P. W., Stratford I. J. RSU 1069, a 2-nitroimidazole containing an alkylating group: high efficiency as a radio- and chemosensitizer in vitro and in vivo. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1653–1656. doi: 10.1016/0360-3016(84)90521-2. [DOI] [PubMed] [Google Scholar]
- Dulhanty A. M., Li M., Whitmore G. F. Isolation of Chinese hamster ovary cell mutants deficient in excision repair and mitomycin C bioactivation. Cancer Res. 1989 Jan 1;49(1):117–122. [PubMed] [Google Scholar]
- Jones N. J., Cox R., Thacker J. Isolation and cross-sensitivity of X-ray-sensitive mutants of V79-4 hamster cells. Mutat Res. 1987 May;183(3):279–286. doi: 10.1016/0167-8817(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Kennedy K. A., Teicher B. A., Rockwell S., Sartorelli A. C. The hypoxic tumor cell: a target for selective cancer chemotherapy. Biochem Pharmacol. 1980 Jan 1;29(1):1–8. doi: 10.1016/0006-2952(80)90235-x. [DOI] [PubMed] [Google Scholar]
- Keyes S. R., Fracasso P. M., Heimbrook D. C., Rockwell S., Sligar S. G., Sartorelli A. C. Role of NADPH:cytochrome c reductase and DT-diaphorase in the biotransformation of mitomycin C1. Cancer Res. 1984 Dec;44(12 Pt 1):5638–5643. [PubMed] [Google Scholar]
- Kirkpatrick D. L. The development of hypoxic tumor cell cytotoxic agents. Pharmacol Ther. 1989;40(3):383–399. doi: 10.1016/0163-7258(89)90086-7. [DOI] [PubMed] [Google Scholar]
- Marshall R. S., Paterson M. C., Rauth A. M. Deficient activation by a human cell strain leads to mitomycin resistance under aerobic but not hypoxic conditions. Br J Cancer. 1989 Mar;59(3):341–346. doi: 10.1038/bjc.1989.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. P., Holtzman J. L. The role of catalytic superoxide formation in the O2 inhibition of nitroreductase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1267–1274. doi: 10.1016/0006-291x(75)90163-1. [DOI] [PubMed] [Google Scholar]
- Robson C. N., Harris A. L., Hickson I. D. Isolation and characterization of Chinese hamster ovary cell lines sensitive to mitomycin C and bleomycin. Cancer Res. 1985 Nov;45(11 Pt 1):5304–5309. [PubMed] [Google Scholar]
- Schlager J. J., Powis G. Mitomycin C is not metabolized by but is an inhibitor of human kidney NAD(P)H: (quinone-acceptor)oxidoreductase. Cancer Chemother Pharmacol. 1988;22(2):126–130. doi: 10.1007/BF00257309. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., O'Neill P., Sheldon P. W., Silver A. R., Walling J. M., Adams G. E. RSU 1069, a nitroimidazole containing an aziridine group. Bioreduction greatly increases cytotoxicity under hypoxic conditions. Biochem Pharmacol. 1986 Jan 1;35(1):105–109. doi: 10.1016/0006-2952(86)90566-6. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Wolf C. R., Workman P. Molecular enzymology of the reductive bioactivation of hypoxic cell cytotoxins. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):983–986. doi: 10.1016/0360-3016(89)90900-0. [DOI] [PubMed] [Google Scholar]
- Wardman P., Clarke E. D. Oxygen inhibition of nitroreductase: electron transfer from nitro radical-anions to oxygen. Biochem Biophys Res Commun. 1976 Apr 19;69(4):942–949. doi: 10.1016/0006-291x(76)90464-2. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Baker M. A., Lemmon M. J., Pearson C. I., Adams J. A., Brown J. M., Lee W. W., Tracy M. Structure-activity relationships for benzotriazine di-N-oxides. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):977–981. doi: 10.1016/0360-3016(89)90899-7. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]


