Abstract
The idea that the rat hippocampus stores a map of space is based on the existence of “place cells” that show “location-specific” firing. The discharge of place cells is confined with remarkable precision to a cell-specific part of the environment called the cell’s “firing field.” We demonstrate here that firing is not nearly as reliable in the time domain as in the positional domain. Discharge during passes through the firing field was compared with a model with Poisson variance of the location-specific firing determined by the time-averaged positional firing rate distribution. Place cells characteristically fire too little or too much compared with expectations from the random model. This fundamental property of place cells is referred to as “excess firing variance” and has three main implications: (i) Place cell discharge is not only driven by the summation of many small, asynchronous excitatory synaptic inputs. (ii) Place cell discharge may encode a signal in addition to the current head location. (iii) The excess firing variance helps explain why the errors in computing the rat’s position from the simultaneous activity of many place cells are large.
How do rodents solve difficult spatial problems? On behavioral grounds, it is believed that rats (and mice) can form map-like representations of their surroundings. Once such a representation is formed, the rat can use it to navigate because the representation contains information about the overall layout or geometry of the surroundings (1–3). Given that a spatial map exists, it is natural to ask how it is organized in the nervous system. That is, where is the map located and how does it operate?
Our current understanding of spatial maps is based on the discovery of hippocampal place cells 25 years ago (4). It was observed that individual neurons in the hippocampus (pyramidal cells of CA3 and CA1) (5) show “location-specific” firing. A given place cell discharges rapidly only when a rat’s head is in a certain part of the environment. Outside this “firing field” region, the cell rarely discharges (Fig. 1A). The precise confinement of discharge to firing fields at once suggested that the hippocampus is a key component of the map and that mapping information is in part positional information. In this view, the rat’s current location is signaled by the conjoint firing of a set of place cells. As the rat moves, its head leaves the field of some cells and enters the field of others, so that the across-cell firing pattern changes in a characteristic way. It thus is presumed that the rat’s head position can be accurately calculated if the current across-cell discharge pattern is known (6, 7).
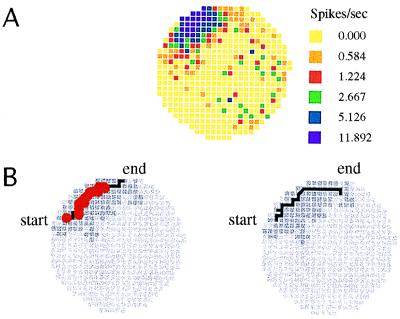
Figure 1.
Time averaged (A) and temporal firing properties (B) of a typical place cell in the CA1 region of the hippocampus. (A) The positional firing rate distribution averaged over 20 min is summarized by a firing rate map in the cylindrical apparatus. The circular colored area indicates the floor area of the cylinder. The firing rate in yellow pixels is exactly zero. The key next to the map indicates the median rate in each color category; darker colors encode higher rates. Virtually all the firing is confined to the firing field, which is the dark area near 11 o’clock. (B) Shown is where the cell fired spikes during two nearly identical passes through the firing field. The field is shown in dark gray, the path through the field is a black line, and the pixels in which spikes occurred are marked by red dots. The initial 1.77 sec of the two passes are 94% “similar.” Similarity was measured by putting the position time series for the two passes into register at the field center and then counting the percentage of the corresponding 1/60th-sec position samples that differed by at most one pixel. Despite the spatial and temporal similarity of the passes, 18 spikes were fired during the 1.77-sec duration pass on the left and no spikes at all were fired during the 2.11-sec pass on the right. Thus, the precise confinement of place cell discharge to firing fields is not accompanied by reliable firing over the behaviorally significant time scale of seconds, the time that it takes rats to go through typical firing fields.
This appealing picture is predicated on a tacit assumption—that in addition to firing only when the head is in the firing field, that a place cell fires in much the same way each time the head goes through the field along much the same path. It is not uncommon, however, for a robust place cell to be silent as the rat’s head passes through the center of its firing field (8). Such failures to discharge are seen even if there was substantial firing on other, nearly identical passes. Fig. 1B shows the discharge along two passes of a rat through the firing field in which the sequence of pixels and time spent in each pixel were closely matched so that position, time at position, instantaneous running speed, average running speed, and direction of running are nearly the same during the two passes. Nevertheless, the cell fired 18 spikes on one pass and no spikes at all on the other.
The purpose of this paper is to demonstrate that such “excess variance” of firing is characteristic of place cells. One way to demonstrate this would be to show that decreases in firing from a maximum are not well predicted by how much a path deviates from the path that produces the maximum firing. Unfortunately, this procedure cannot be used because the differences between pairs of passes do not satisfy the triangular inequality requirement of a metric that estimates the difference between two passes. Thus, there is no unambiguous way to decide how much two paths differ and therefore no way to predict the firing along one path from the firing along another.
A stochastic method therefore was used to characterize the excess variance. Specifically, the number of spikes observed during complete passes through the firing field was compared with the number of spikes expected from the time-averaged firing rates in the sequence of pixels encountered during the pass. A pass begins when the rat’s head enters the firing field and ends when the head leaves the field. Thus, we neglected all processes that occur on a time scale shorter than a pass, which is about a second. In doing so, we ignored at least one known source of firing modulation, namely, the 5–10 Hz theta rhythm (9, 10). This omission is justified because a pass lasts many theta cycles, so the effects of theta modulation average out.
What constraints are there on the method of predicting the number of spikes during a pass using only the sequence of head positions and the time-averaged firing rates at those positions? Because we are interested in demonstrating the excess variance, we chose the model that produces the greatest variance of spike number for any given pass. This choice maximizes the difficulty of rejecting the null hypothesis, that the observed firing is predictable from head position and the time averaged positional rate pattern.
This decision dictates the use of an inhomogeneous Poisson process (IPP). In general, an IPP is a time series of ordinary Poisson processes such that the rate parameter for each ordinary Poisson process is adjusted according to the current state of the system. For instance, an IPP can be used to estimate the number of events registered by a radiation detector as the position of the detector is moved relative a radiation source. In the present case, the rate parameter for each ordinary Poisson process in the IPP is set to the time-averaged firing rate at the current head location. The IPP was chosen because it yields the greatest possible variance of spike occurrence for identical passes through the field of any model in which firing depends only on the mean firing rate at each position.†
METHODS
The behavioral and electrophysiological procedures and basic place cell analyses are described in detail elsewhere (8). Briefly, male Long Evans rats were anesthetized with Nembutal (40 mg/kg) and implanted with a driveable bundle of 25 μm nichrome electrodes. Recording sessions between 16 and 64 min in duration were made while a rat collected food pellets randomly scattered into a cylinder 76 cm in diameter and 51 cm high. The inside wall was uniformly gray except for a white card that covered 90° of arc. For one rat the cylinder contained instead one white card and one black card, each covering 45° of arc and separated (center to center) by 135° of arc.
The position of a light-emitting diode mounted on the rat’s head was detected and stored at 60 Hz. The time series of filtered (300–10,000 Hz) action potentials was simultaneously recorded and stored. Offline, the time-averaged firing rate in each 3 cm × 3 cm pixel visited by the rat was calculated as the number of spikes discharged in the pixel divided by the total time spent there. A firing field was defined as a set of pixels such that the firing rate in each pixel was >0 spikes/sec and such that each pixel in the field shared at least one side with another pixel in the field. Only the largest field is considered for each cell if more that one field was found. The field center was defined as the 3×3 group of pixels with the greatest mean rate.
A pass through the firing field was defined as the time series of positions starting when the light-emitting diode (LED) entered the field and ending when the LED left the field. To enhance the reliability of firing rate estimates passes were studied only if they met several criteria: (i) Each pass had to last at least 1 sec. (ii) The head light had to be detected for every 1/60-sec sample. (iii) The pass could include only pixels visited for at least 0.25 sec during recording. (iv) The pass had to go through the field center.
The observed number of spikes fired during a pass was compared with the number of spikes predicted from the session-averaged positional firing rate distribution. The predicted activity during a pass depends only on the specific pixels visited and the time spent in those pixels without regard to the sequence of positions.
For a given pass, the number of spikes is given by,
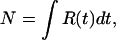 |
1 |
where R(t) is the instantaneous firing rate at each time step (t) along the pass. Under the assumption that the mean firing rate in the current pixel estimates R(t), a discrete form of the model can be written that uses the mean firing rates at positions along the path to circumvent difficulties of estimating instantaneous firing rates.
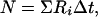 |
2 |
where Ri is the time-averaged firing rate at position i in the pass through the field and Δt, the sample duration, is 1/60th sec. The distribution of N is Poisson with variance and mean = N.
How likely is it that the observed number of spikes (S) for a pass is a random deviation from the model predicting the expected number N? If N > 4, the Poisson distribution can be approximated by a normal distribution with mean and variance N. We therefore can calculate Z, the standard normal deviate for S as:
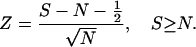 |
3a |
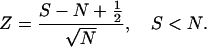 |
3b |
Z is the number of SDs that separate the observed and expected number of spikes for a single pass. Decreasing S − N by 1/2 reduces Z to correct for transforming from a discrete to a continuous probability distribution.
Eqs. 3a and 3b preserve the sign of Z. Z > 0 for passes in which S > N and Z < 0 for passes in which S < N. If Z ≥ 1.96, the probability that the observed number of spikes is consistent with the model is ≤0.05.
Deciding whether deviations of the observed number of spikes from Poisson expectations are characteristically large must be based on the distribution of the deviations. To tell whether place cells routinely discharge “too many” or “too few” spikes on single passes we examined the distribution of Z values for a large number of passes. If the variance of the Z values is broader than the variance of the unit normal distribution, the null hypothesis that place cell discharge reliably reflects head position can be rejected.
The analytic calculation was adapted from ref. 11 and converges precisely with Monte Carlo simulations in which a random number generator is used to decide, for each small time interval, whether a spike occurred. In such a calculation, the probability of a spike for a short interval is set from the firing rate in the current pixel. The “unit interval” was 1/10th of the 16.7-msec sample time to make the probability of getting more than 1 spike in an interval very small. The Monte Carlo calculation then proceeds in the following way: Imagine that the rate in a pixel is 20 spikes/sec. In 1/60th sec, the mean number of expected spikes is 0.333 and in a unit interval is 0.0333. A random number is generated for each unit interval and the number of spikes is counted. Now, the rat either stays in the same pixel for the next 1/60th sec or it moves. If it stays, the probability of a spike is kept the same; if it moves, the probability is changed accordingly. This process is repeated for all samples to yield a total number of spikes. Repeating the Monte Carlo calculation 100,000 times for each pass produces a distribution of integers. Now it is possible to calculate the probability that the observed number of spikes was a random selection from the distribution. We found that the mean and variance of the distribution of the expected number of spikes was virtually identical using the analytic and the Monte Carlo methods.
RESULTS
A total of 51 cells were examined. Of these, 31 cells satisfied the criteria that the firing rate in the field center had to be at least 10 spikes/sec and that the rat had to make at least 15 passes through the field center.
In a second pruning step, the 31 cells were tested for a systematic drift of the Z scores during the recording time. Such a drift could occur, for example, because the amplitude of the cell’s waveform decreased during the session. In this case, early passes would be associated with higher firing rates and later passes with lower firing rates. Consequently, Z scores higher than expected from time average rates would occur early in the session, and Z scores lower than expected would occur late in the session. To detect drift, we calculated the linear regression coefficient for the time sequence of Z scores during each session (robs). Next, the sequence of Z scores was shuffled 1,000 times and the linear regression coefficient was calculated for each, thus removing any systematic time trend. Finally, robs was compared with the distribution of the regression values for the shuffled sequences. If robs was in the extreme 5% of this distribution the trend was considered significant (12, 13). On this basis, five cells were eliminated from further consideration, leaving 26 cells for the complete analysis.
The observed sequence of Z scores for a unit recorded for 48 min is shown in Fig. 2. Because the gray band indicates the 95% region of confidence, it is clear that many more Z scores are outside the band than would be expected by chance. In addition, it is clear that there is no major upward or downward trend for the Z scores. Moreover, Z scores that are too high and too low according to the IPP often occur near each other, a direct indication that the Z score distribution does not reflect a simple time trend. No significant runs were detected in the sequence of Z scores from any of the 26 cells. The proximity of excessively low and high Z scores is typical of each cell in the 26-cell sample.
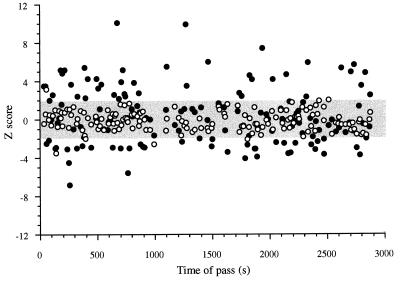
Figure 2.
A plot of the time series of Z scores (•) for the observed firing on all passes through the field of a place cell recorded for 2,880 sec (48 min). The gray band shows the 95% confidence interval. Note that the number of Z scores that lie outside the 95% band are much greater than expected by chance. To emphasize the improbability of the occurrence of observed Z scores, ○ show the time series of Z scores for single Monte Carlo simulations of the number of spikes expected on each pass through the field; these expected values lie almost entirely within the 95% band. Critically, there is no trend for the observed Z scores to increase or decrease with time; the correlation between observed Z score and time was −0.08 (P > 0.05). Note that high negative and positive Z scores often follow each other and do so over the whole recording time. This is an independent indication that the excess variance is not because of a tendency of a large number of spikes to be generated per pass at one time during the session and small numbers to be generated per pass at another time.
Z scores were calculated for 1,440 passes through the fields of the 26 cells (mean duration 5 sec), for an average of 55 passes per cell. Because the average recording duration was 30 min per cell, the rat went through the field center about 1.8 times per min. The distribution of the Z scores was compared with the unit normal distribution (Fig. 3). The mean of the Z scores is 0.18, which is reliably different from 0.0 [t = 2.8; df = 1,439; P(t ≥ 2.8) = 0.005]. More importantly, however, the distribution of Z scores is much broader that the unit normal distribution. Because the observed variance is 5.9, it is extremely unlikely that the Z scores come from a process with a unit normal distribution [F1439,∞ = 5.9; P(F ≥ 5.9) < 10−220]. The excess variance is seen for paths from individual cells as well. From F-tests, the probability that the variance could arise from the model was P < 0.001 for 22 cells, 0.001 < P < 0.05 for two cells, and P > 0.05 for only two cells. It is therefore extremely unlikely that the observed distribution of Z scores has an underlying unit normal distribution. Thus, the mean positional firing rates of individual place cells poorly estimate the spike activity during single passes of the head through the firing field. Furthermore, this discharge cannot be generated by an IPP or by any other, more restrictive process that depends only on the current location of the head.
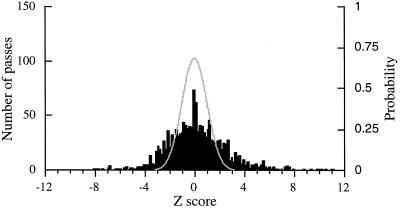
Figure 3.
A histogram shows the distribution of Z scores for 1,440 passes through firing fields (left ordinate). The expected distribution is the unit normal distribution drawn in red (right ordinate). The variance of the observed distribution is more than 5 times greater than 1. The F-test comparing the observed variance to 1 indicates that the probability of this outcome is virtually zero. Note also that large peak of observed Z scores near zero indicates that the distribution of observed Z scores is not normal.
What other origins of the excess variance are possible? One is that hippocampal pyramidal cells fire “complex spikes” as well as ordinary, simple spikes (14–16). A complex spike is a series of extracellularly recorded action potentials with decrementing amplitude. The first component of a complex-spike is the same as a simple spike. To be part of a complex-spike, each successive component had to occur within 10 msec of the preceding component. We counted each component as a separate spike. Excess variance then might arise if place cells tended to fire complex-spikes on some passes and only simple spikes on other passes. To test for this variance, we eliminated all components except the first from each complex-spike and recomputed the time-averaged positional rate distribution. This left field shape and position intact but reduced the number of spikes and therefore field size by about 30%. After recalculating the passes, the excess variance was still apparent [F510,∞ = 3.2; P (F ≥ 3.2) < 10−37]. The excess variance still occurred if complex spikes were completely eliminated, removing a total of about 50% of the original action potentials. Thus, complex-spiking does not account for the excess variance.
Another possible basis for the excess variance concerns the exact properties of passes. As shown above, extreme differences in discharge can be seen for very similar passes. Nevertheless, there might be, for instance, a trend for some cells to fire rapidly and other cells to fire slowly on passes when the rat runs fast. Accordingly, we identified for each cell passes in which the number of spikes was either unexpectedly low or unexpectedly high (Z < −1.96 or Z > 1.96; the 0.05 probability tails). We then compared for each cell the running speed during passes with “too few” spikes to the running speed during passes with “too many” spikes. From t tests, running speed differences classified according to expected spike counts reached the 0.05 level of probability for 3/26 cells. This proportion of 0.115 is not detectably higher than the proportion of 0.05 of the cells that would be expected to yield the 0.05 probability level by chance alone (Z = 0.767; P = 0.221). Similar separations according to unexpectedly low and high Z scores were done for the total time spent in the field during the pass, for the distance moved in the field, path direction, and for tortuosity [the ratio of the distance traveled in the field to the line segment between the field entry and exit locations (17)]. In no case was there a trend that accounted for the excess variance. [Head direction was not analyzed in this way because the complicated shapes of paths make it impossible to generate a meaningful average head direction for an entire pass. Earlier work shows, however, that place cell discharge is independent of head direction in open fields (18).]
The final possibility we considered is that excess variance is caused by changes in the overall behavioral state of the animal. For example, place cells might fire rapidly if the rat was using external cues for navigation and more slowly if it was using self-motion information. In this case, the activity of place cells with overlapping or coincident fields would be expected to covary. We find, however, that Z scores are uncorrelated for simultaneously recorded cell pairs. Two example cell pairs are shown in Fig. 4. The slope of the regression line was 0.06 for the cell pair in Fig. 4A and −0.05 for the cell pair in Fig. 4B. The correlation of Z scores was calculated for nine simultaneously recorded cell pairs with overlapping fields. The range of the regression line slopes was −0.11 to 0.19. The mean slope was 0.08, which is not reliably different from 0 [t = 1.76; df = 8; P(t ≥ 1.76) = 0.12], indicating that the tendency of the two cells to fire more rapidly or slowly than expected from the IPP are independent of each other. It also should be noted that for some cell pairs (see Fig. 4B) there seemed to be extra points along the 45° line in addition to the uncorrelated points. Further analysis of the duration, distance, running speed, path direction, and tortuosity of such paths did not reveal any way in which passes whose scatter points lay on the 45° line differed from other passes.
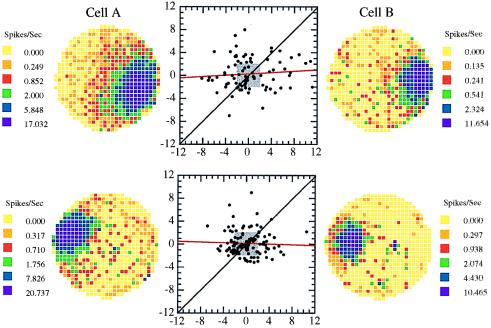
Figure 4.
Scattergrams for the Z scores for two pairs of simultaneously recorded cells with overlapping firing fields. Each point represents the Z score for each cell of a pair on a single pass through the field. If the excess variance were caused by any overall state of the animal, the Z scores of the cell pairs should be correlated with each other. In fact, the approximately circular patterns of points in the scattergrams and the near-zero slope of the regression lines (red) (upper: r = 0.06, n.s; lower: r = −0.05, n.s) indicate that the processes responsible for the excess variance of each cell in a pair are independent. The small gray boxes show the limits of the 95% confidence intervals. Note that many Z scores for all four cells in this figure lie outside the 95% confidence region. Interestingly, there appears to be a rather large number of points along the 45° line for the bottom pair of cells. These correlated pairs seem to occur only at low Z scores and correspond to the extra values near zero in the histogram of Fig. 3.
The lack of correlation for cell pairs is in contrast to the observation that cells that fire in one reference frame tend to be silent during the activity of cells that fire in other reference frames (19). This effect would lead to points along the −45° line rather than to uncorrelated points or points along the 45° line.
DISCUSSION
The IPP used to predict the number of spikes was chosen because it yields the maximum variance of spike number for any purely positional generator of place cell firing. Our basic finding was that the observed firing variance greatly exceeds expectations from the IPP. We call this basic place cell characteristic “excess variance.” We now consider the implications of excess variance for three aspects of the organization and properties of place cells.
Neuronal Mechanisms Underlying Place Cells.
The fact that the cells often fire too much or too little puts major constraints on the neuronal mechanisms that drive place cell activity. On the one hand, excess variance may be caused by a gating mechanism that prevents cells from firing or forces them to fire very rapidly on certain occasions. An anatomical substrate for gating exists in the form of the dual inputs to CA1 from CA3 and from entorhinal cortex (EC). If, for example, the EC > CA1 input generates feedforward inhibition (20–22), increased activity of layer III cells in EC could cause CA1 place cells to be too quiet or even silent. Note that this proposed role of the EC input would require great specificity of connections for the excess variance to be uncorrelated for cells with fields in the same part of the apparatus. A second basis for excess variance would be for place cells to be driven by powerful synchronous inputs. In this view, the motorneuron model for converting synaptic drive into firing frequency is inapplicable to place cells because it is implausible that the excess variance could arise from the smooth summation of many small, asynchronous excitatory postsynaptic potentials. A similar conclusion was reached about synaptic drive onto neocortical neurons although the time scale was much briefer than whole seconds (23).
The Place Cell Signal.
The excess variance suggests that we must abandon the appealing notion that place cells exclusively and reliably signal the rat’s position by firing rate. If place cells signal only position, the excess variance means that they are quite unreliable in the time domain. Although excess variance indeed may be just a form of noise, that is a hard conclusion to accept given the great positional precision of place cell firing.
The alternative conclusion is that in addition to current position place cells carry a signal that is yet to be characterized. The notion is that the excess variance represents modulation of the positional signal that our initial explorations did not uncover. Consider again those place cells with overlapping or coincident firing fields in a certain part of the apparatus. Perhaps some of these cells fire mainly when the rat approaches the target region along a certain trajectory and others fire mainly when the rat approaches along a different trajectory. Just as well the particular cells that fire on a certain pass might indicate where the rat will wind up after exiting the field. These possibilities are in line with a vector field model of hippocampal mapping (24) in which the firing of place cells during locomotion is biased locally in that direction that eventually will take the rat to a goal. Provision for multiple goals in a single environment is made by supposing that different place cells with different local directional biases are turned on or off by gain control depending on which potential goal is current. Preliminary tests of this hypothesis were made by once again examining the properties of passes through firing fields grouped according to Z scores. Although our examination was by no means exhaustive, we could see no characteristic that was common to high or low rate paths. Nevertheless, because no place or set of places was a consistent goal during pellet chasing, our analysis should be repeated for passes through fields on the central platform of an eight-arm maze. One then could ask if certain cells fired at higher rates if the rat left a certain arm or if it was in the process of going to a certain arm.
A related issue concerns the difficulty of testing whether the excess variance arises because individual cells are “tuned” to very specific behavioral states. Data from cells with overlapping fields imply that the state of the place cell population does not vary in conjunction with any specific state of the animal. Nevertheless, individual cells could signal very particular behavioral states as was suggested in early studies (25, 26). Such proposals run into the grave problem that they are virtually impossible to falsify. Consider first which variables to examine. As the number of variables goes up, the number of possible distinct states for the animal increases as a product. For example, if only position is important, experience tells us that dividing an apparatus into about 500 equal-size pixels gives a good picture of the positional firing pattern. If head direction also is included, it should be resolved to one part in 20 so the number of distinguishable states is now 10,000. What about running speed? Step length? The nature of the current gait? It would take an enormous amount of data gathering to fill such multidimensional arrays. Furthermore, there is no end to the possible variables that might be included. Which ones are reasonable is a matter of judgment and taste. We have developed a video-based method that could reveal which variables are important as long as they are at the behavioral level (27), but it remains for future work to apply this method to the excess variance problem. We stress, however, that the analysis of simultaneously recorded cells with coincident fields provides a nearly perfect way of ruling out global states of the animal as causing the variance. Thus, the firing of both cells cannot be controlled by whether the animal is paying attention, is happy, is eating, and so on. Perhaps some cells are tuned to attention and others to inattention, some are tuned to happiness and others to sadness, but hypotheses of this sort are frivolous unless there is some independent reason for proposing them. As stated above, the excess variance may reflect a physiological mode of hippocampal activity and not an unknown message.
Computing Position from Place Cell Activity.
Finally, we raise the issue of computing the rat’s position given the conjoint activity of a set of place cells. It seems clear that reconstruction of position should be less accurate by using the real spike time series of a certain number of place cells compared with spike time series generated for the same number of place cells using an IPP. (It also seems clear that the accuracy of position reconstruction from real place cell firing can be improved by increasing the number of cells.)
To compare real and IPP-generated spike trains, we used data from multiple simultaneously recorded place cells provided by Matthew Wilson and Bruce McNaughton (6). This is the data set from rat 1 from ref. 6. We used 34 of the 76 complex-spike cells; these are the cells considered to exhibit statistically significant spatial information content (6). A time-averaged firing rate distribution was made for each cell. To determine the position of the rat from the real data, the time sequence of positions was replayed. A firing rate vector averaged for 50 msec (the time resolution of rat tracking) then was calculated for the 34 cells. The projection of this momentary rate vector onto the session-averaged rate vector for each pixel was calculated and the rat’s position was taken as the pixel in which the projection was largest. The computation was done only for intervals during which the length of momentary rate vector was not zero (intervals during which at least one cell fired). The error was the distance between the computed and tracked positions. The error was averaged over all 50 msec intervals for 13.4 min of data. The error then was found when the rate was averaged for intervals of 100, 150, 200, … , 2,000 msec. Finally, the same process was used to compute the error when “firing” was generated by the IPP instead of the observed spike discharge.
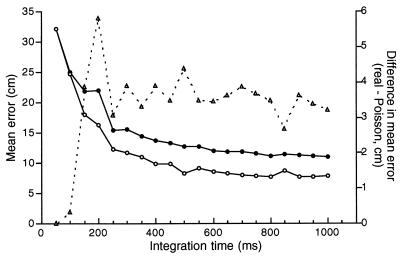
The error calculations are summarized in Fig. 5. The spike trains from the IPP consistently yielded smaller mean errors for averaging times greater than 100 msec. We believe that the poorer positional prediction from the real data reflects the excess variance. We imagine that the nearly constant difference at averaging times >300 msec is caused by displacements of the rat during the averaging interval; such errors will be worse for longer intervals and will apply with equal strength to computations from the real and IPP-generated data. We also imagine that the errors are nearly equal for the real and IPP-based spike trains at 50 and 100 msec averaging times because it is unlikely, given the time-averaged firing rates, that more than one cell will be “active” in any sample interval. Accordingly, the predicted error is expected to be the same for each process. The fact that the error difference is greatest at 200 msec is interesting because this time is a “natural” integrating time; it is about equal to the cycle time of the theta rhythm of the hippocampal electroencephalogram.
Figure 5.
Error of the predicted position of a rat’s head from the simultaneous firing of 34 place cells as a function of the integration time for calculating firing rate. The error was calculated in two ways. The line with ○ was obtained on the assumption that discharge is generated by an IPP whose mean value is set by the time-averaged firing rate of each place cell at the rat’s current position. The line with • shows the error when head location is calculated using the real spike time series. The difference (Errorreal − ErrorIPP) (▵) shows that the IPP always does at least as well as the real spikes. At an integration time of 200 msec, the difference in error is about 6 cm, an improvement of about 35%. We attribute the lower prediction accuracy from the real data to excess variance.
In summary, the excess variance firing described here is a second surprising property of the temporal firing patterns of place cells, the first being the precession of place cell discharge relative to the phase of the hippocampal electroencephalogram “theta” rhythm that accompanies locomotion (9, 10). The possibility that the excess variance indicates a nonpositional signal is of great potential importance—such a signal would provide a major clue to the nature of mapping computations carried on by the hippocampus.
Acknowledgments
We are grateful to Matt Stead, John Kubie, Jim Ranck, and Petr Lansky for helpful discussions. This work was supported by National Institutes of Health Grant NS 20686.
ABBREVIATION
- IPP
inhomogeneous Poisson process
Footnotes
A commentary on this article begins on page 2717.
A preliminary version of this paper was presented at the 1995 Annual Meeting of the Society for Neuroscience (San Diego, CA, Nov. 11–16, 1995).
References
- 1.Tolman E C. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 2.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 3.Gallistel C R. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- 4.O’Keefe J, Dostrovsky J. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 5.Ranck J B., Jr Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 6.Wilson M A, McNaughton B L. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 7.McHugh T J, Blum K I, Tsien J Z, Tonegawa S, Wilson M A. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- 8.Muller R U, Kubie J L, Ranck J B., Jr J Neurosci. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Keefe J, Recce M. Hippocampus. 1993;3:317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 10.Skaggs W E, McNaughton B L, Wilson M A, Barnes C A. Hippocampus. 1996;6:149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 11.Cox D R, Lewis P A W. The Statistical Analysis of Series of Events. London: Methuen; 1968. [Google Scholar]
- 12.Edgington E S, Bland B H. J Neurosci Methods. 1993;47:169–177. doi: 10.1016/0165-0270(93)90079-7. [DOI] [PubMed] [Google Scholar]
- 13.Dayhoff J E, Gerstein G L. J Neurophysiol. 1983;49:1334–1348. doi: 10.1152/jn.1983.49.6.1334. [DOI] [PubMed] [Google Scholar]
- 14.Fox S E, Ranck J B., Jr Exp Neurol. 1975;49:299–313. doi: 10.1016/0014-4886(75)90213-7. [DOI] [PubMed] [Google Scholar]
- 15.Fox S E, Ranck J B., Jr Exp Brain Res. 1981;41:399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- 16.Pavlides C, Winson J. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arolfo M P, Nerad L, Schenk F, Bures J. Behav Neurosci. 1994;108:308–316. doi: 10.1037//0735-7044.108.2.308. [DOI] [PubMed] [Google Scholar]
- 18.Muller R U, Bostock E, Taube J, Kubie J L. J Neurosci. 1994;14:7235–7251. doi: 10.1523/JNEUROSCI.14-12-07235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gothard K M, Skaggs W E, Moore K M, McNaughton B L. J Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Empson R M, Heinemann U. Hippocampus. 1995;5:104–107. doi: 10.1002/hipo.450050203. [DOI] [PubMed] [Google Scholar]
- 21.Soltesz I. Hippocampus. 1995;5:120–124. doi: 10.1002/hipo.450050206. [DOI] [PubMed] [Google Scholar]
- 22.Buzsaki G, Penttonen M, Bragin A, Nadasady Z, Chrobak J. Hippocampus. 1995;5:141–146. doi: 10.1002/hipo.450050210. [DOI] [PubMed] [Google Scholar]
- 23.Softky W R, Koch C. J Neurosci. 1993;13:334–350. doi: 10.1523/JNEUROSCI.13-01-00334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerstner W, Abbott L F. J Comput Neurosci. 1996;4:79–94. doi: 10.1023/a:1008820728122. [DOI] [PubMed] [Google Scholar]
- 25.Ranck J B., Jr Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 26.O’Keefe J. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 27.Fenton A A, Muller R U. J Neurosci Methods. 1996;70:211–227. doi: 10.1016/s0165-0270(96)00082-9. [DOI] [PubMed] [Google Scholar]