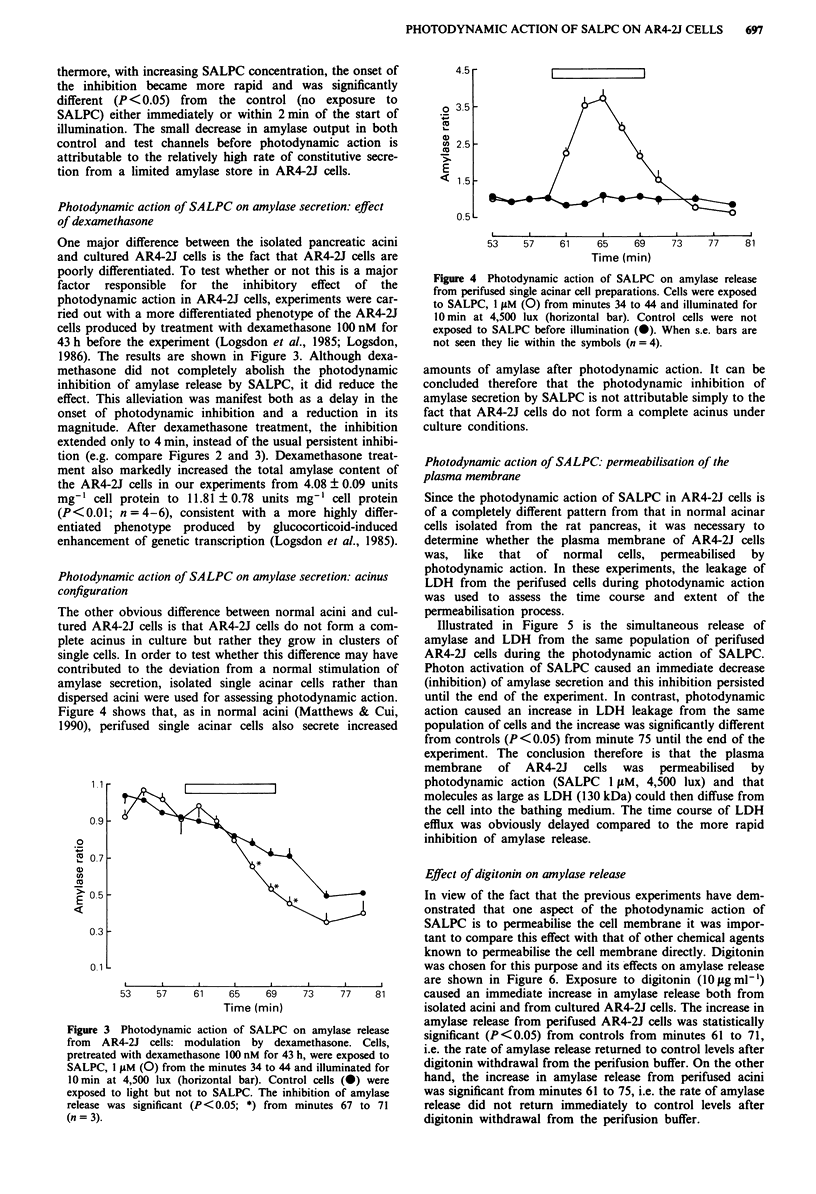
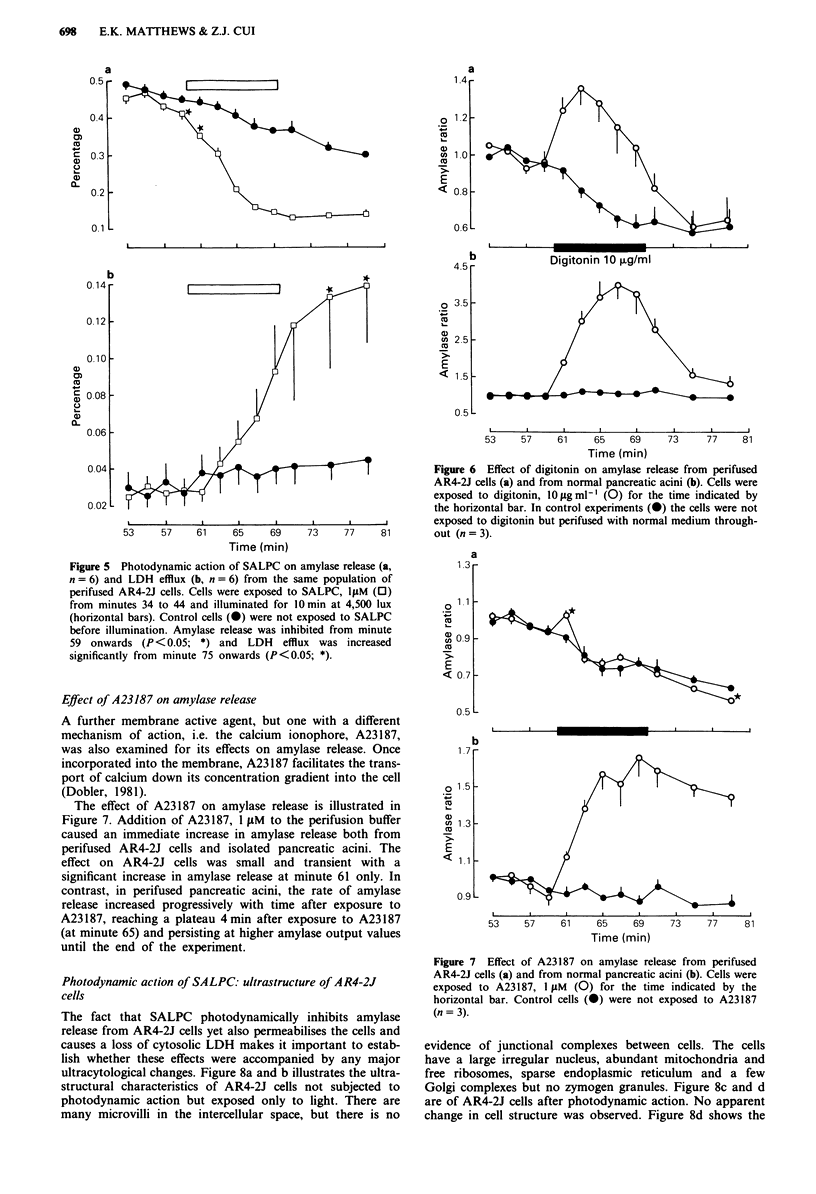
Abstract
The photodynamic effects of sulphonated aluminium phthalocyanine (SALPC) have been compared on cultured AR4-2J cells of a pancreatic carcinoma cell line and on exocrine cells of the normal phenotype freshly isolated from the rat pancreas; a multi-channel perifusion system was used for this kinetic study in vitro. Whereas light alone or SALPC alone was without effect on either cell type, photon activation of cellularly-bound SALPC with light greater than 570 nm permeabilised the cells and caused an increase in amylase secretion from normal acinar cells but a dose-dependent inhibition (10(-7) to 10(-5) M) of amylase release from AR4-2J cells. In contrast, direct permeabilisation of the plasma membrane with digitonin, 10 micrograms ml-1, evoked a marked release of amylase from both types of cell. Elevation of [Ca2+]i by the ionophore A23187, 10(-6) M, elicited secretion of amylase from normal cells but had little effect on AR4-2J cells. Finally, it was established that the differential photodynamic effects of SALPC on amylase release were not attributable to any topographical differences in the microanatomical organisation of normal or tumour-derived cells; furthermore, the structural integrity of normal and AR4-2J cells was maintained after the photodynamic action of SALPC. It is concluded that the generation of singlet oxygen is responsible for permeabilisation of both types of cell and that photon-activated SALPC has functionally distinct effects on the constitutive secretion of amylase of tumour cells and the regulated secretory pathway of normal cells. These observations may be important in the development of drugs with a selective photodynamic action on pancreatic tumour cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Solomon T. E., Jamieson J. D. Sequential dissociation of the exocrine pancreas into lobules, acini, and individual cells. Methods Cell Biol. 1978;20:361–378. doi: 10.1016/s0091-679x(08)62028-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Ceska M., Birath K., Brown B. A new and rapid method for the clinical determination of alpha-amylase activities in human serum and urine. Optimal conditions. Clin Chim Acta. 1969 Dec;26(3):437–444. doi: 10.1016/0009-8981(69)90071-0. [DOI] [PubMed] [Google Scholar]
- Chan W. S., Svensen R., Phillips D., Hart I. R. Cell uptake, distribution and response to aluminium chloro sulphonated phthalocyanine, a potential anti-tumour photosensitizer. Br J Cancer. 1986 Feb;53(2):255–263. doi: 10.1038/bjc.1986.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W. The subcellular localization of immunoglobulin in mouse plasma cells, as studied with immunoferritin cytochemistry on ultrathin frozen sections. Am J Anat. 1980 Jun;158(2):161–169. doi: 10.1002/aja.1001580206. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D. Glucocorticoids increase cholecystokinin receptors and amylase secretion in pancreatic acinar AR42J cells. J Biol Chem. 1986 Feb 15;261(5):2096–2101. [PubMed] [Google Scholar]
- Logsdon C. D., Moessner J., Williams J. A., Goldfine I. D. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985 Apr;100(4):1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker D. S., Lilja H. S., French J., Kuhlmann E., Noll W. Transplantation of azaserine-induced carcinomas of pancreas in rats. Cancer Lett. 1979 Aug;7(4):197–202. doi: 10.1016/s0304-3835(79)80080-4. [DOI] [PubMed] [Google Scholar]
- Manyak M. J., Russo A., Smith P. D., Glatstein E. Photodynamic therapy. J Clin Oncol. 1988 Feb;6(2):380–391. doi: 10.1200/JCO.1988.6.2.380. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Cui Z. J. Photodynamic action of rose bengal on isolated rat pancreatic acini: stimulation of amylase release. FEBS Lett. 1989 Oct 9;256(1-2):29–32. doi: 10.1016/0014-5793(89)81711-9. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Mesler D. E. Photodynamic effects of erythrosine on the smooth muscle cells of guinea-pig taenia coli. Br J Pharmacol. 1984 Oct;83(2):555–566. doi: 10.1111/j.1476-5381.1984.tb16520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rogers J., Hughes R. G., Matthews E. K. Cyclic GMP inhibits protein kinase C-mediated secretion in rat pancreatic acini. J Biol Chem. 1988 Mar 15;263(8):3713–3719. [PubMed] [Google Scholar]
- Swarovsky B., Steinhilber W., Scheele G. A., Kern H. F. Coupled induction of exocrine proteins and intracellular compartments involved in the secretory pathway in AR4-2J cells by glucocorticoids. Eur J Cell Biol. 1988 Oct;47(1):101–111. [PubMed] [Google Scholar]
- Tralau C. J., Barr H., Sandeman D. R., Barton T., Lewin M. R., Bown S. G. Aluminum sulfonated phthalocyanine distribution in rodent tumors of the colon, brain and pancreas. Photochem Photobiol. 1987 Nov;46(5):777–781. doi: 10.1111/j.1751-1097.1987.tb04847.x. [DOI] [PubMed] [Google Scholar]
- Viguerie N., Estève J. P., Susini C., Logsdon C. D., Vaysse N., Ribet A. Dexamethasone effects on somatostatin receptors in pancreatic acinar AR4-2J cells. Biochem Biophys Res Commun. 1987 Sep 30;147(3):942–948. doi: 10.1016/s0006-291x(87)80161-4. [DOI] [PubMed] [Google Scholar]
- Viguerie N., Tahiri-Jouti N., Esteve J. P., Clerc P., Logsdon C., Svoboda M., Susini C., Vaysse N., Ribet A. Functional somatostatin receptors on a rat pancreatic acinar cell line. Am J Physiol. 1988 Jul;255(1 Pt 1):G113–G120. doi: 10.1152/ajpgi.1988.255.1.G113. [DOI] [PubMed] [Google Scholar]
- Weishaupt K. R., Gomer C. J., Dougherty T. J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976 Jul;36(7 Pt 1):2326–2329. [PubMed] [Google Scholar]
- Womack M. D., Hanley M. R., Jessell T. M. Functional substance P receptors on a rat pancreatic acinar cell line. J Neurosci. 1985 Dec;5(12):3370–3378. doi: 10.1523/JNEUROSCI.05-12-03370.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonuschot G., Matthews E. K., Corps A. N., Metcalfe J. C. Permeabilization of thymocytes by photon activation of erythrosin. FEBS Lett. 1987 Mar 23;213(2):401–405. doi: 10.1016/0014-5793(87)81530-2. [DOI] [PubMed] [Google Scholar]



