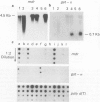
Abstract
Increased expression of the mdr1 gene, encoding the 175 kDa P-glycoprotein, and the gst-pi gene, encoding the anionic isozyme of glutathione S-transferase (GST), have previously been detected in continuous human breast cancer cell lines selected in vitro for resistance to doxorubicin. In this present study we have measured RNA levels of mdr1 and gst-pi in primary human breast tumour biopsies prior to chemotherapy and from tumours which have different inherent responses to doxorubicin treatment, including colon, head and neck squamous cell carcinomas and myeloid leukaemias. Detectable levels of mdr1 mRNA was observed in 25 out of 49 breast tumours, with up to a 100-fold range in expression. A narrower range of gst-pi expression has also been observed in these tumours. Chemosensitivity of cells grown in short-term culture from some of the breast tumours has been measured by an in vitro colony forming assay in the presence of doxorubicin. Comparison of the dose of doxorubicin causing 50% inhibition of growth (ID50) with RNA levels showed that the tumours with high mdr1 expression had high ID50, while the more sensitive explants had low mdr1 expression. These results support a role for mdr1 gene expression in determining the response of human breast cancer cells to chemotherapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blum R. H., Carter S. K. Adriamycin. A new anticancer drug with significant clinical activity. Ann Intern Med. 1974 Feb;80(2):249–259. doi: 10.7326/0003-4819-80-2-249. [DOI] [PubMed] [Google Scholar]
- Bonadonna G., Monfardini S., De Lena M., Fossati-Bellani F., Beretta G. Phase I and preliminary phase II evaluation of adriamycin (NSC 123127). Cancer Res. 1970 Oct;30(10):2572–2582. [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Cowan K. H., Batist G., Tulpule A., Sinha B. K., Myers C. E. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9328–9332. doi: 10.1073/pnas.83.24.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffie A. M., Alam T., Seneviratne C., Beenken S. W., Batra J. K., Shea T. C., Henner W. D., Goldenberg G. J. Multifactorial resistance to adriamycin: relationship of DNA repair, glutathione transferase activity, drug efflux, and P-glycoprotein in cloned cell lines of adriamycin-sensitive and -resistant P388 leukemia. Cancer Res. 1988 Jul 1;48(13):3595–3602. [PubMed] [Google Scholar]
- Fink D. W., Jr, Mirkin B. L. Effects of chemical sympathectomy in neonatal and adult mice on C-1300 neuroblastoma tumor growth and catecholamine content. Cancer Res. 1987 Nov 1;47(21):5620–5625. [PubMed] [Google Scholar]
- Fojo A. T., Shen D. W., Mickley L. A., Pastan I., Gottesman M. M. Intrinsic drug resistance in human kidney cancer is associated with expression of a human multidrug-resistance gene. J Clin Oncol. 1987 Dec;5(12):1922–1927. doi: 10.1200/JCO.1987.5.12.1922. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. H., Bell D. R., Karakousis C., Slocum H. K., Kartner N., Rustum Y. M., Ling V., Baker R. M. P-glycoprotein in human sarcoma: evidence for multidrug resistance. J Clin Oncol. 1987 Sep;5(9):1452–1460. doi: 10.1200/JCO.1987.5.9.1452. [DOI] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Guild B. C., Mulligan R. C., Gros P., Housman D. E. Retroviral transfer of a murine cDNA for multidrug resistance confers pleiotropic drug resistance to cells without prior drug selection. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1595–1599. doi: 10.1073/pnas.85.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B. Hybridization of oligo(dT) to RNA on nitrocellulose. Gene Anal Tech. 1987 Mar-Apr;4(2):17–22. doi: 10.1016/0735-0651(87)90013-6. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B. The glutathione S-transferases: a group of multifunctional detoxification proteins. Adv Enzymol Relat Areas Mol Biol. 1978;46:383–414. doi: 10.1002/9780470122914.ch6. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kaye S. B. The multidrug resistance phenotype. Br J Cancer. 1988 Dec;58(6):691–694. doi: 10.1038/bjc.1988.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P., Amtmann E., Sauer G. The simultaneous extraction of high-molecular-weight DNA and of RNA from solid tumors. Anal Biochem. 1983 Oct 15;134(2):288–294. doi: 10.1016/0003-2697(83)90299-3. [DOI] [PubMed] [Google Scholar]
- Merkel D. E., Fuqua S. A., Tandon A. K., Hill S. M., Buzdar A. U., McGuire W. L. Electrophoretic analysis of 248 clinical breast cancer specimens for P-glycoprotein overexpression or gene amplification. J Clin Oncol. 1989 Aug;7(8):1129–1136. doi: 10.1200/JCO.1989.7.8.1129. [DOI] [PubMed] [Google Scholar]
- Moscow J. A., Fairchild C. R., Madden M. J., Ransom D. T., Wieand H. S., O'Brien E. E., Poplack D. G., Cossman J., Myers C. E., Cowan K. H. Expression of anionic glutathione-S-transferase and P-glycoprotein genes in human tissues and tumors. Cancer Res. 1989 Mar 15;49(6):1422–1428. [PubMed] [Google Scholar]
- Moscow J. A., Townsend A. J., Goldsmith M. E., Whang-Peng J., Vickers P. J., Poisson R., Legault-Poisson S., Myers C. E., Cowan K. H. Isolation of the human anionic glutathione S-transferase cDNA and the relation of its gene expression to estrogen-receptor content in primary breast cancer. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6518–6522. doi: 10.1073/pnas.85.17.6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryan R. M., Baker L. H., Gottlieb J. E., Rivkin S. E., Balcerzak S. P., Grumet G. N., Salmon S. E., Moon T. E., Hoogstraten B. Dose response evaluation of adriamycin in human neoplasia. Cancer. 1977 May;39(5):1940–1948. doi: 10.1002/1097-0142(197705)39:5<1940::aid-cncr2820390505>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- O'Bryan R. M., Luce J. K., Talley R. W., Gottlieb J. A., Baker L. H., Bonadonna G. Phase II evaluation of adriamycin in human neoplasia. Cancer. 1973 Jul;32(1):1–8. doi: 10.1002/1097-0142(197307)32:1<1::aid-cncr2820320101>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Safa A. R. Photoaffinity labeling of the multidrug-resistance-related P-glycoprotein with photoactive analogs of verapamil. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7187–7191. doi: 10.1073/pnas.85.19.7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon S. E., Grogan T. M., Miller T., Scheper R., Dalton W. S. Prediction of doxorubicin resistance in vitro in myeloma, lymphoma, and breast cancer by P-glycoprotein staining. J Natl Cancer Inst. 1989 May 3;81(9):696–701. doi: 10.1093/jnci/81.9.696. [DOI] [PubMed] [Google Scholar]
- Scotto K. W., Biedler J. L., Melera P. W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science. 1986 May 9;232(4751):751–755. doi: 10.1126/science.2421411. [DOI] [PubMed] [Google Scholar]
- Shen D. W., Fojo A., Chin J. E., Roninson I. B., Richert N., Pastan I., Gottesman M. M. Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification. Science. 1986 May 2;232(4750):643–645. doi: 10.1126/science.3457471. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Hackett A. J., Lan S., Stampfer M. R. Use of an efficient method for culturing human mammary epithelial cells to study adriamycin sensitivity. Cancer Chemother Pharmacol. 1981;6(3):237–244. doi: 10.1007/BF00256976. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Lan S., Ceriani R., Hackett A. J., Stampfer M. R. Clonal proliferation of cultured nonmalignant and malignant human breast epithelia. Cancer Res. 1981 Nov;41(11 Pt 1):4637–4643. [PubMed] [Google Scholar]
- Smith H. S., Lippman M. E., Hiller A. J., Stampfer M. R., Hackett A. J. Response to doxorubicin of cultured normal and cancerous human mammary epithelial cells. J Natl Cancer Inst. 1985 Feb;74(2):341–347. [PubMed] [Google Scholar]
- Stallard S., Morrison J. G., George W. D., Kaye S. B. Distribution of doxorubicin to normal breast and tumour tissue in patients undergoing mastectomy. Cancer Chemother Pharmacol. 1990;25(4):286–290. doi: 10.1007/BF00684887. [DOI] [PubMed] [Google Scholar]
- Ueda K., Cardarelli C., Gottesman M. M., Pastan I. Expression of a full-length cDNA for the human "MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987 May;84(9):3004–3008. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Clark D. P., Chen C. J., Roninson I. B., Gottesman M. M., Pastan I. The human multidrug resistance (mdr1) gene. cDNA cloning and transcription initiation. J Biol Chem. 1987 Jan 15;262(2):505–508. [PubMed] [Google Scholar]
- Van der Bliek A. M., Baas F., Van der Velde-Koerts T., Biedler J. L., Meyers M. B., Ozols R. F., Hamilton T. C., Joenje H., Borst P. Genes amplified and overexpressed in human multidrug-resistant cell lines. Cancer Res. 1988 Nov 1;48(21):5927–5932. [PubMed] [Google Scholar]