Abstract
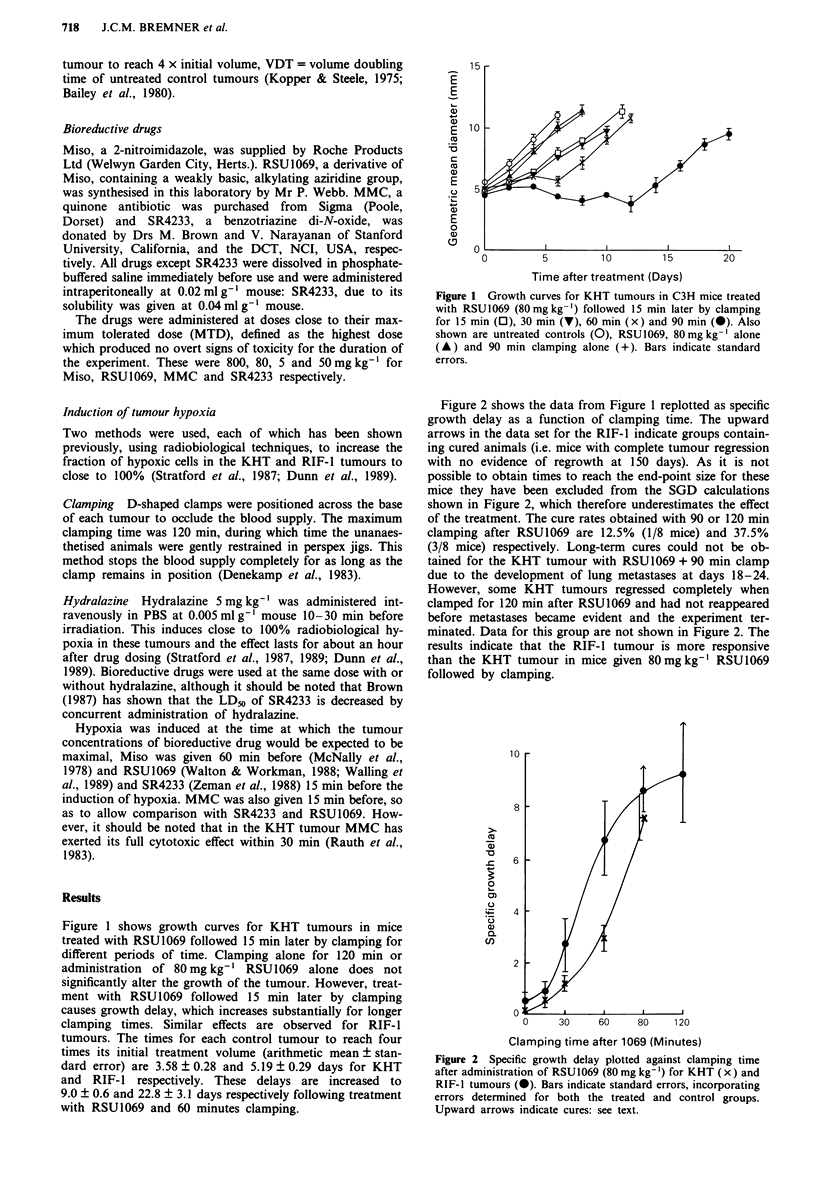
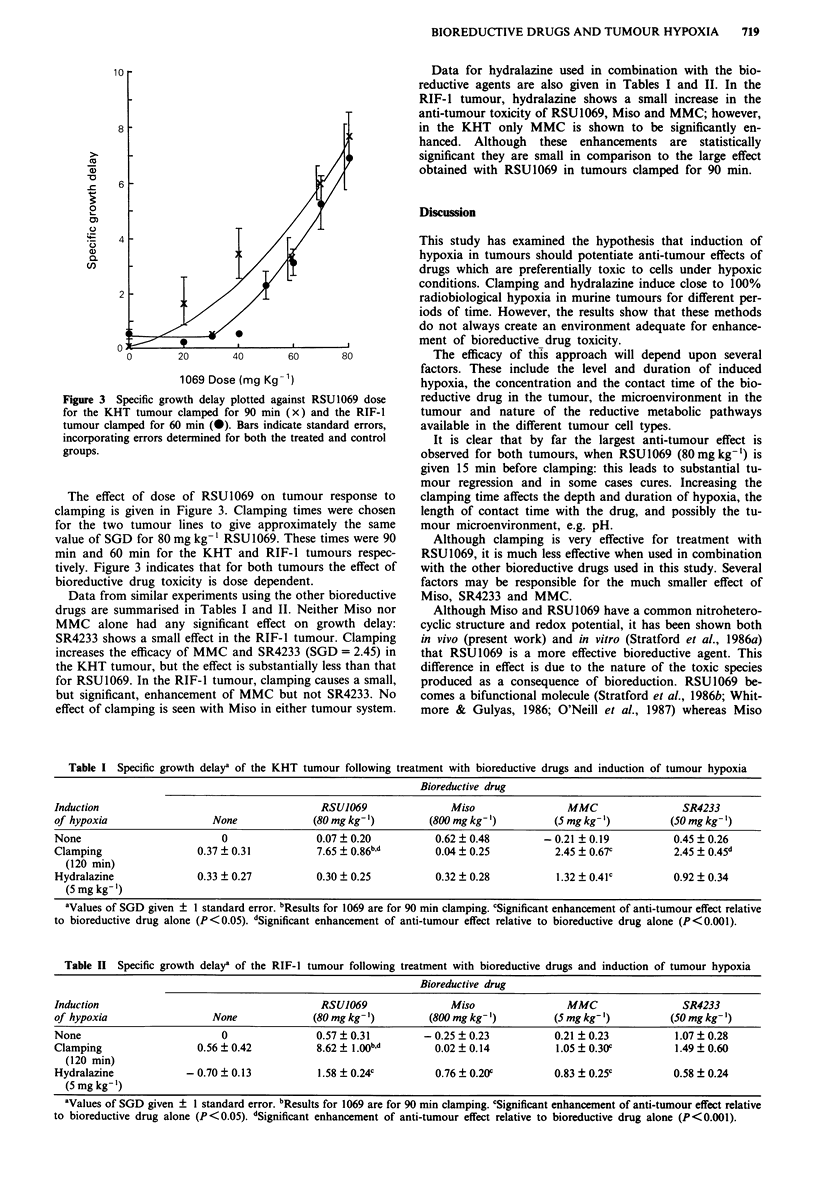
In this work tumour hypoxia is induced by physically occluding the tumour vascular supply by clamping, or by giving mice 5 mg kg-1 hydralazine. These methods have previously been shown to increase the radiobiological hypoxic fraction in tumours close to 100%. Their effectiveness in potentiating the bioreductive toxicity of: misonidazole (800 mg kg-1), RSU1069 (80 mg kg-1), mitomycin C (5 mg kg-1) and SR4233 (50 mg kg-1) is assessed in the RIF-1 and KHT tumours using regrowth delay as an assay. Clamping alone for 120 min gives little or no response, but when RSU1069 is administered 15 min before clamping, large growth delays result. RIF-1 tumours clamped for 90 or 120 min with RSU1069 give cure rates of 12.5% and 37.5% respectively. Less effect with clamping is seen for the other bioreductive agents. The effect of hydralazine with RSU1069 although significant in the RIF-1 tumour, is modest compared to that for clamping. Small enhancements of toxicity are seen with hydralazine in combination with misonidazole in the RIF-1 tumour and mitomycin C in both tumours. The varying effectiveness of these treatments is attributed to several factors which include the level and duration of hypoxia, concentration and contact time of the bioreductive drugs, the microenvironment of the tumour and the nature of the reductive metabolic pathways available in the different tumour cell types.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M. J., Gazet J. C., Smith I. E., Steel G. G. Chemotherapy of human breast-carcinoma xenografts. Br J Cancer. 1980 Oct;42(4):530–536. doi: 10.1038/bjc.1980.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. The effect of hydralazine on the tumor cytotoxicity of the hypoxic cell cytotoxin RSU-1069: evidence for therapeutic gain. Int J Radiat Oncol Biol Phys. 1987 Apr;13(4):579–585. doi: 10.1016/0360-3016(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Durand R. E., Olive P. L. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1279–1282. doi: 10.1016/0360-3016(86)90153-7. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Hill S. A., Hobson B. Vascular occlusion and tumour cell death. Eur J Cancer Clin Oncol. 1983 Feb;19(2):271–275. doi: 10.1016/0277-5379(83)90426-1. [DOI] [PubMed] [Google Scholar]
- Dulhanty A. M., Li M., Whitmore G. F. Isolation of Chinese hamster ovary cell mutants deficient in excision repair and mitomycin C bioactivation. Cancer Res. 1989 Jan 1;49(1):117–122. [PubMed] [Google Scholar]
- Dunn J. F., Frostick S., Adams G. E., Stratford I. J., Howells N., Hogan G., Radda G. K. Induction of tumour hypoxia by a vasoactive agent. A combined NMR and radiobiological study. FEBS Lett. 1989 Jun 5;249(2):343–347. doi: 10.1016/0014-5793(89)80655-6. [DOI] [PubMed] [Google Scholar]
- Keyes S. R., Fracasso P. M., Heimbrook D. C., Rockwell S., Sligar S. G., Sartorelli A. C. Role of NADPH:cytochrome c reductase and DT-diaphorase in the biotransformation of mitomycin C1. Cancer Res. 1984 Dec;44(12 Pt 1):5638–5643. [PubMed] [Google Scholar]
- Kirkpatrick D. L. The development of hypoxic tumor cell cytotoxic agents. Pharmacol Ther. 1989;40(3):383–399. doi: 10.1016/0163-7258(89)90086-7. [DOI] [PubMed] [Google Scholar]
- Kopper L., Steel G. G. The therapeutic response of three human tumor lines maintained in immune-suppressed mice. Cancer Res. 1975 Oct;35(10):2704–2713. [PubMed] [Google Scholar]
- Marshall R. S., Paterson M. C., Rauth A. M. Deficient activation by a human cell strain leads to mitomycin resistance under aerobic but not hypoxic conditions. Br J Cancer. 1989 Mar;59(3):341–346. doi: 10.1038/bjc.1989.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally N. J., Denekamp J., Sheldon P., Flockhart I. R., Stewart F. A. Radiosensitization by misonidazole (Ro 07-0582). The importance of timing and tumor concentration of sensitizer. Radiat Res. 1978 Mar;73(3):568–580. [PubMed] [Google Scholar]
- O'Neill P., McNeil S. S., Jenkins T. C. Induction of DNA crosslinks in vitro upon reduction of the nitroimidazole-aziridines RSU-1069 and RSU-1131. Biochem Pharmacol. 1987 Jun 1;36(11):1787–1792. doi: 10.1016/0006-2952(87)90239-5. [DOI] [PubMed] [Google Scholar]
- Rauth A. M., Mohindra J. K., Tannock I. F. Activity of mitomycin C for aerobic and hypoxic cells in vitro and in vivo. Cancer Res. 1983 Sep;43(9):4154–4158. [PubMed] [Google Scholar]
- Rice G. C., Hoy C., Schimke R. T. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5978–5982. doi: 10.1073/pnas.83.16.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUIT H. D., SHALEK R. J. RESPONSE OF SPONTANEOUS MAMMARY CARCINOMA OF THE C3H MOUSE TO X IRRADIATION GIVEN UNDER CONDITIONS OF LOCAL TISSUE ANOXIA. J Natl Cancer Inst. 1963 Sep;31:497–509. [PubMed] [Google Scholar]
- Stratford I. J., Adams G. E., Godden J., Howells N. Induction of tumour hypoxia post-irradiation: a method for increasing the sensitizing efficiency of misonidazole and RSU 1069 in vivo. Int J Radiat Biol. 1989 Mar;55(3):411–422. doi: 10.1080/09553008914550451. [DOI] [PubMed] [Google Scholar]
- Stratford I. J., Adams G. E., Godden J., Nolan J., Howells N., Timpson N. Potentiation of the anti-tumour effect of melphalan by the vasoactive agent, hydralazine. Br J Cancer. 1988 Aug;58(2):122–127. doi: 10.1038/bjc.1988.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford I. J., Walling J. M., Silver A. R. The differential cytotoxicity of RSU 1069: cell survival studies indicating interaction with DNA as a possible mode of action. Br J Cancer. 1986 Mar;53(3):339–344. doi: 10.1038/bjc.1986.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. M. Selective chemotherapy of noncycling cells in an in vitro tumor model. Cancer Res. 1974 Dec;34(12):3501–3503. [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I., Guttman P. Response of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer. 1981 Feb;43(2):245–248. doi: 10.1038/bjc.1981.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B. A., Lazo J. S., Sartorelli A. C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981 Jan;41(1):73–81. [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Varghese A. J., Whitmore G. F. Modification of guanine derivatives by reduced 2-nitroimidazoles. Cancer Res. 1983 Jan;43(1):78–82. [PubMed] [Google Scholar]
- Walling J. M., Deacon J., Holliday S., Stratford I. J. High uptake of RSU 1069 and its analogues melanotic melanomas. Cancer Chemother Pharmacol. 1989;24(1):28–32. doi: 10.1007/BF00254101. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Wolf C. R., Workman P. Molecular enzymology of the reductive bioactivation of hypoxic cell cytotoxins. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):983–986. doi: 10.1016/0360-3016(89)90900-0. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Workman P. Pharmacokinetics and metabolism of the mixed-function hypoxic cell sensitizer prototype RSU 1069 in mice. Cancer Chemother Pharmacol. 1988;22(4):275–281. doi: 10.1007/BF00254231. [DOI] [PubMed] [Google Scholar]
- Whitmore G. F., Gulyas S. Studies on the toxicity of RSU-1069. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1219–1222. doi: 10.1016/0360-3016(86)90262-2. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Brown J. M., Lemmon M. J., Hirst V. K., Lee W. W. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- Zeman E. M., Hirst V. K., Lemmon M. J., Brown J. M. Enhancement of radiation-induced tumor cell killing by the hypoxic cell toxin SR 4233. Radiother Oncol. 1988 Jul;12(3):209–218. doi: 10.1016/0167-8140(88)90263-0. [DOI] [PubMed] [Google Scholar]


