Abstract
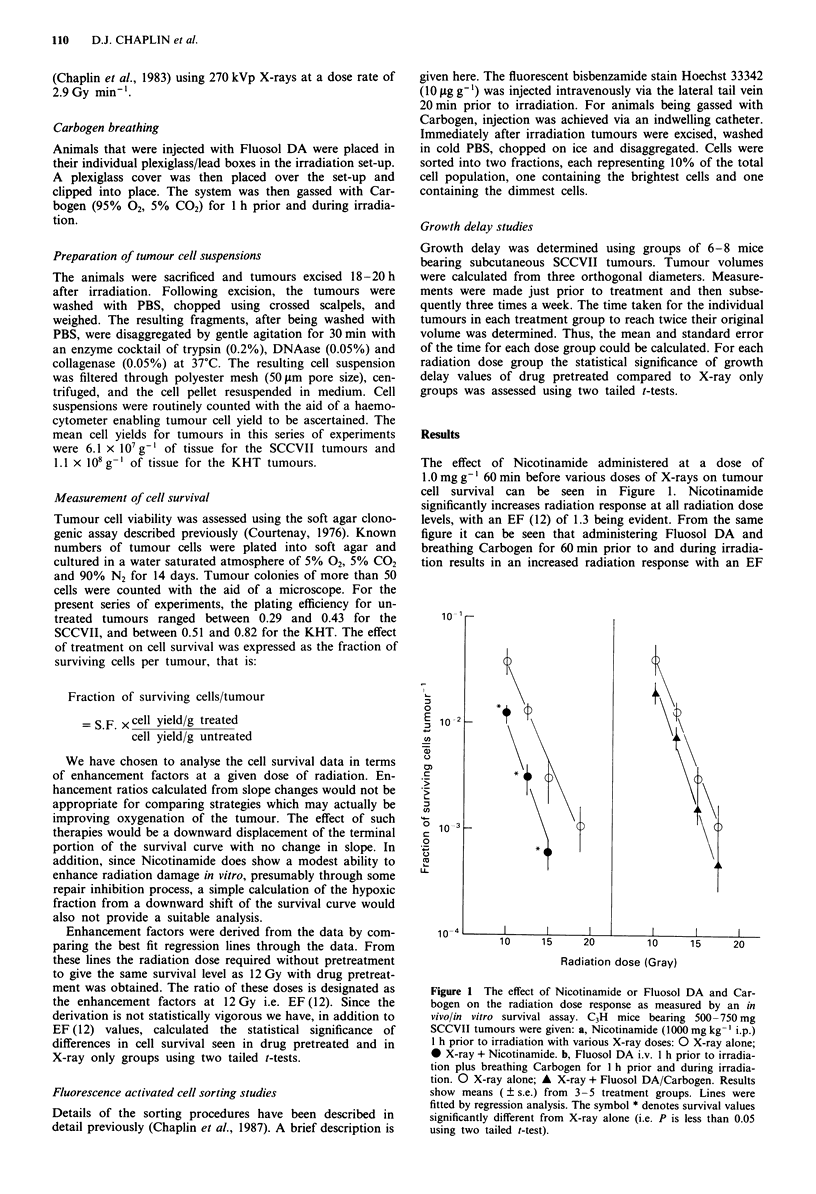
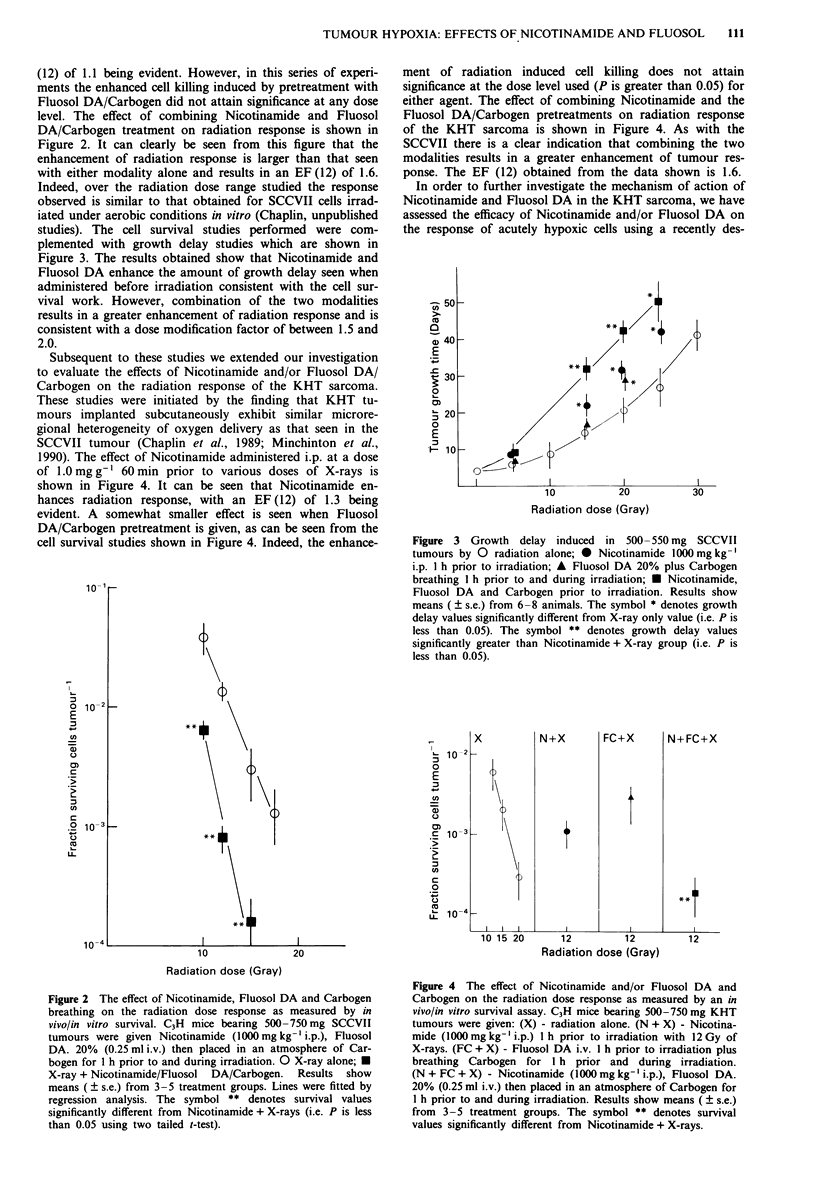
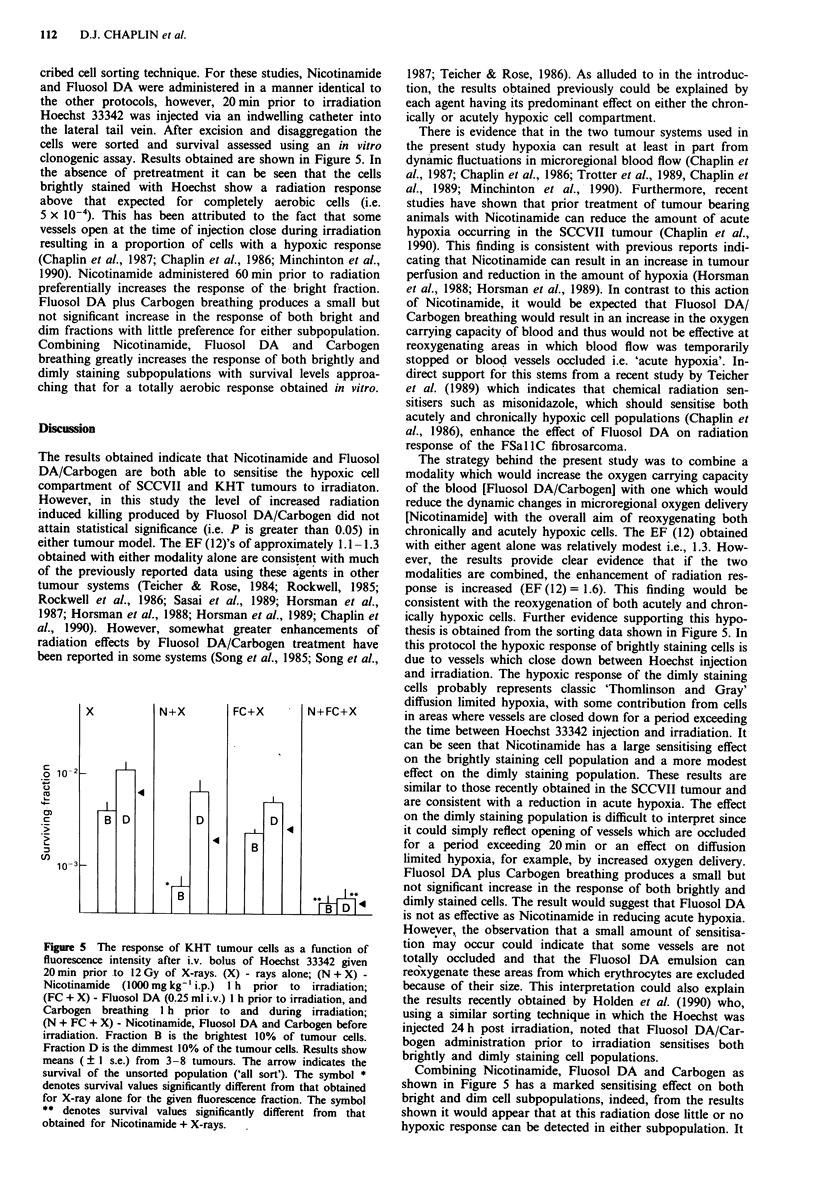
The effect of Nicotinamide and/or treatment with Fluosol DA and Carbogen breathing on the radiation response of 500-750 mg SCCVII and KHT tumours has been evaluated. Pretreatment with Fluosol DA/Carbogen or Nicotinamide resulted in relatively modest enhancements of radiation damage with enhancement factors of 1.1 and 1.3 being observed using an in vivo/in vitro clonogenic end-point. A combination of Nicotinamide and Fluosol DA/Carbogen resulted in a larger enhancement factor of 1.6 over the radiation dose ranges studied. These modification factors reflect a value close to that expected for a fully aerobic response in this survival range. Growth delay studies in the SCCVII tumour provided similar results. Using a recently developed fluorescence activated cell sorting technique, which utilizes the in vivo pharmacokinetic and DNA binding properties of the bisbenzamide stain Hoechst 33342, the effect of Nicotinamide and/or Fluosol DA/Carbogen schedules on the occurrence of acute hypoxia was assessed. The results clearly show that Nicotinamide significantly reduces the amount of 'acute hypoxia', but has a lesser effect on 'chronic' hypoxic cells. However, combinations of Nicotinamide and Fluosol DA/Carbogen significantly increase the response of both 'acutely' and 'chronically hypoxic' cells. The results provide evidence that a combination of Nicotinamide and Fluosol DA/Carbogen can provide an effective way of reoxygenating both acutely and chronically hypoxic cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979 Aug;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- Brown J. M. Keynote address: hypoxic cell radiosensitizers: where next? Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):987–993. doi: 10.1016/0360-3016(89)90901-2. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Acker B. D., Horsman M. R. Reduction of tumour blood flow by vasoactive drugs: a role in cancer therapy. Biomed Biochim Acta. 1989;48(2-3):S264–S268. [PubMed] [Google Scholar]
- Chaplin D. J., Durand R. E., Olive P. L. Acute hypoxia in tumors: implications for modifiers of radiation effects. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1279–1282. doi: 10.1016/0360-3016(86)90153-7. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Horsman M. R., Trotter M. J. Effect of nicotinamide on the microregional heterogeneity of oxygen delivery within a murine tumor. J Natl Cancer Inst. 1990 Apr 18;82(8):672–676. doi: 10.1093/jnci/82.8.672. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987 Jan 15;47(2):597–601. [PubMed] [Google Scholar]
- Chaplin D. J., Sheldon P. W., Stratford I. J., Ahmed I., Adams G. E. Radiosensitization in vivo: a study with an homologous series of 2-nitroimidazoles. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Oct;44(4):387–398. doi: 10.1080/09553008314551331. [DOI] [PubMed] [Google Scholar]
- Coleman C. N. Hypoxia in tumors: a paradigm for the approach to biochemical and physiologic heterogeneity. J Natl Cancer Inst. 1988 May 4;80(5):310–317. doi: 10.1093/jnci/80.5.310. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D. A soft agar colony assay for Lewis lung tumour and B16 melanoma taken directly from the mouse. Br J Cancer. 1976 Jul;34(1):39–45. doi: 10.1038/bjc.1976.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dische S. Keynote address: hypoxic cell sensitizers: clinical developments. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):1057–1060. doi: 10.1016/0360-3016(89)90915-2. [DOI] [PubMed] [Google Scholar]
- Guichard M. Chemical manipulations of tissue oxygenation for therapeutic benefit. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1125–1130. doi: 10.1016/0360-3016(89)90266-6. [DOI] [PubMed] [Google Scholar]
- Hirst D. G. Oxygen delivery to tumors. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1271–1277. doi: 10.1016/0360-3016(86)90152-5. [DOI] [PubMed] [Google Scholar]
- Holden S. A., Herman T. S., Teicher B. A. Addition of a hypoxic cell selective cytotoxic agent (mitomycin C or porfiromycin) to Fluosol-DA/carbogen/radiation. Radiother Oncol. 1990 May;18(1):59–70. doi: 10.1016/0167-8140(90)90023-p. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Brown J. M., Hirst V. K., Lemmon M. J., Wood P. J., Dunphy E. P., Overgaard J. Mechanism of action of the selective tumor radiosensitizer nicotinamide. Int J Radiat Oncol Biol Phys. 1988 Sep;15(3):685–690. doi: 10.1016/0360-3016(88)90312-4. [DOI] [PubMed] [Google Scholar]
- Horsman M. R., Chaplin D. J., Brown J. M. Radiosensitization by nicotinamide in vivo: a greater enhancement of tumor damage compared to that of normal tissues. Radiat Res. 1987 Mar;109(3):479–489. [PubMed] [Google Scholar]
- Horsman M. R., Chaplin D. J., Brown J. M. Tumor radiosensitization by nicotinamide: a result of improved perfusion and oxygenation. Radiat Res. 1989 Apr;118(1):139–150. [PubMed] [Google Scholar]
- Intaglietta M., Myers R. R., Gross J. F., Reinhold H. S. Dynamics of microvascular flow in implanted mouse mammary tumours. Bibl Anat. 1977;(15 Pt 1):273–276. [PubMed] [Google Scholar]
- Jonsson G. G., Kjellén E., Pero R. W., Cameron R. Radiosensitization effects of nicotinamide on malignant and normal mouse tissue. Cancer Res. 1985 Aug;45(8):3609–3614. [PubMed] [Google Scholar]
- Kjellén E., Jonsson G. G., Pero R. W., Christensson P. I. Effects of hyperthermia and nicotinamide on DNA repair synthesis, ADP-ribosyl transferase activity, NAD+ and ATP pools, and cytotoxicity in gamma-irradiated human mononuclear leukocytes. Int J Radiat Biol Relat Stud Phys Chem Med. 1986 Jan;49(1):151–162. doi: 10.1080/09553008514552321. [DOI] [PubMed] [Google Scholar]
- Minchinton A. I., Durand R. E., Chaplin D. J. Intermittent blood flow in the KHT sarcoma--flow cytometry studies using Hoechst 33342. Br J Cancer. 1990 Aug;62(2):195–200. doi: 10.1038/bjc.1990.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold H. S., Blachiwiecz B., Blok A. Oxygenation and reoxygenation in 'sandwich' tumours. Bibl Anat. 1977;(15 Pt 1):270–272. [PubMed] [Google Scholar]
- Rockwell S., Mate T. P., Irvin C. G., Nierenburg M. Reactions of tumors and normal tissues in mice to irradiation in the presence and absence of a perfluorochemical emulsion. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1315–1318. doi: 10.1016/0360-3016(86)90162-8. [DOI] [PubMed] [Google Scholar]
- Rockwell S. Use of a perfluorochemical emulsion to improve oxygenation in a solid tumor. Int J Radiat Oncol Biol Phys. 1985 Jan;11(1):97–103. doi: 10.1016/0360-3016(85)90367-0. [DOI] [PubMed] [Google Scholar]
- Song C. W., Lee I., Hasegawa T., Rhee J. G., Levitt S. H. Increase in pO2 and radiosensitivity of tumors by Fluosol-DA (20%) and carbogen. Cancer Res. 1987 Jan 15;47(2):442–446. [PubMed] [Google Scholar]
- Song C. W., Zhang W. L., Pence D. M., Lee I., Levitt S. H. Increased radiosensitivity of tumors by perfluorochemicals and carbogen. Int J Radiat Oncol Biol Phys. 1985 Oct;11(10):1833–1836. doi: 10.1016/0360-3016(85)90041-0. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Franko A. J. On the nature of the radiobiologically hypoxic fraction in tumors. Int J Radiat Oncol Biol Phys. 1980 Jan;6(1):117–120. doi: 10.1016/0360-3016(80)90215-1. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock I. F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972 Jul;45(535):515–524. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Herman T. S., Holden S. A., Jones S. M. Addition of misonidazole, etanidazole, or hyperthermia to treatment with fluosol-DA/carbogen/radiation. J Natl Cancer Inst. 1989 Jun 21;81(12):929–934. doi: 10.1093/jnci/81.12.929. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Rose C. M. Effects of dose and scheduling on growth delay of the Lewis lung carcinoma produced by the perfluorochemical emulsion, Fluosol-DA. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1311–1313. doi: 10.1016/0360-3016(86)90161-6. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Rose C. M. Perfluorochemical emulsions can increase tumor radiosensitivity. Science. 1984 Mar 2;223(4639):934–936. doi: 10.1126/science.6695191. [DOI] [PubMed] [Google Scholar]
- Thomas C., Lartigau E., Malaise E. P., Guichard M. New high O2 carrying perfluorochemical emulsions and/or carbogen: reactions of a human tumor xenograft to irradiation. Int J Radiat Oncol Biol Phys. 1989 May;16(5):1157–1160. doi: 10.1016/0360-3016(89)90273-3. [DOI] [PubMed] [Google Scholar]
- Trotter M. J., Chaplin D. J., Durand R. E., Olive P. L. The use of fluorescent probes to identify regions of transient perfusion in murine tumors. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):931–934. doi: 10.1016/0360-3016(89)90889-4. [DOI] [PubMed] [Google Scholar]


