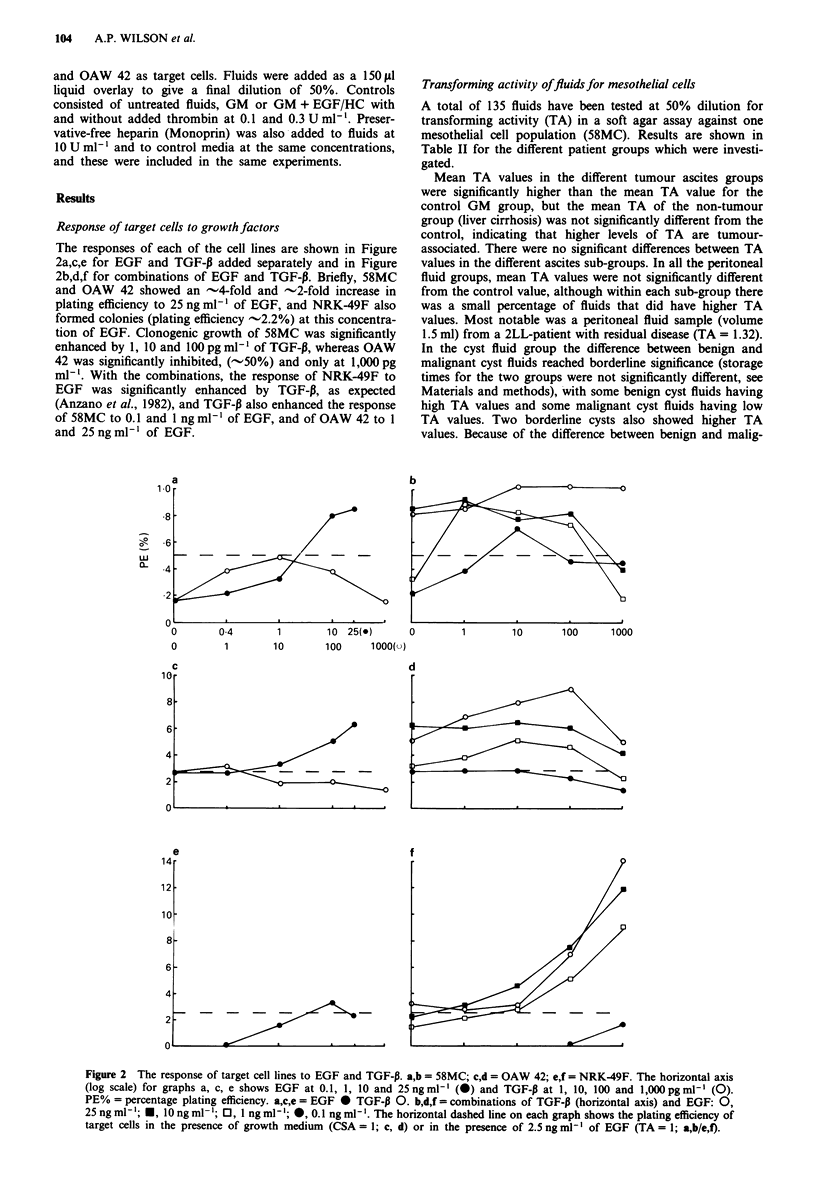
Abstract
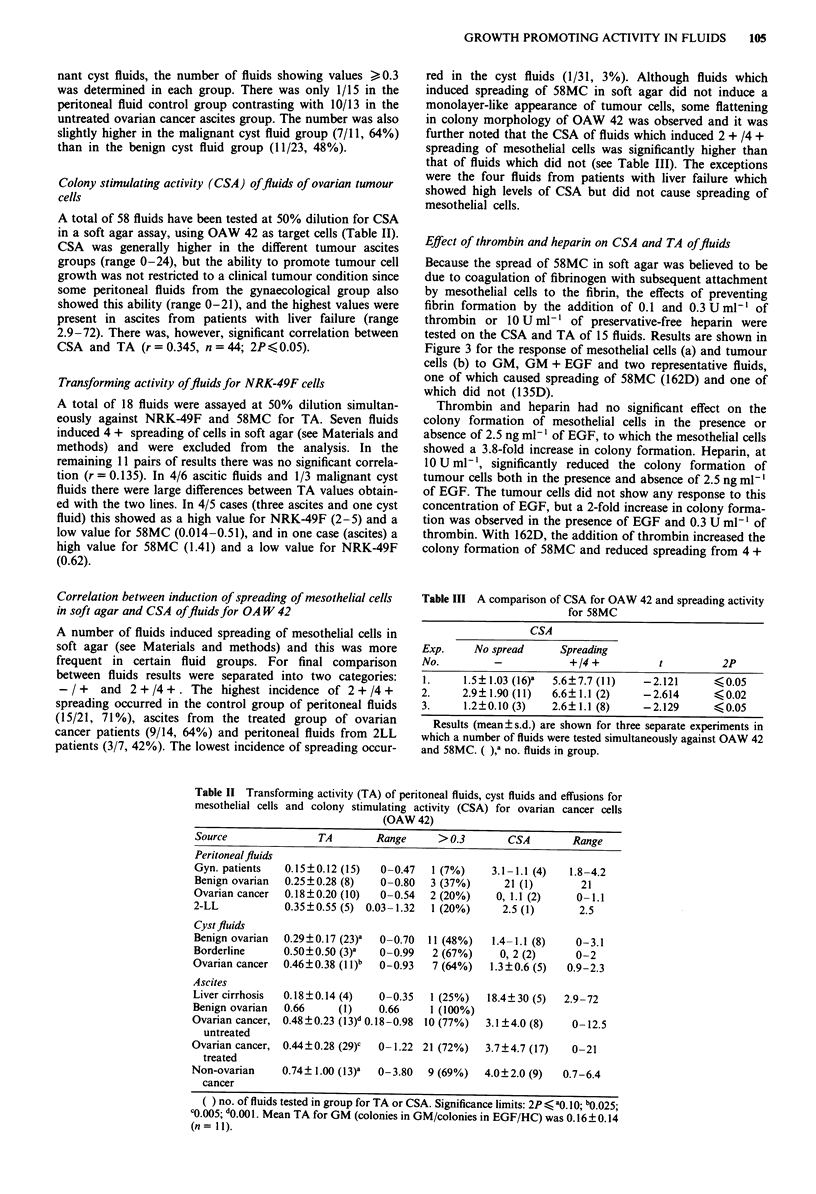
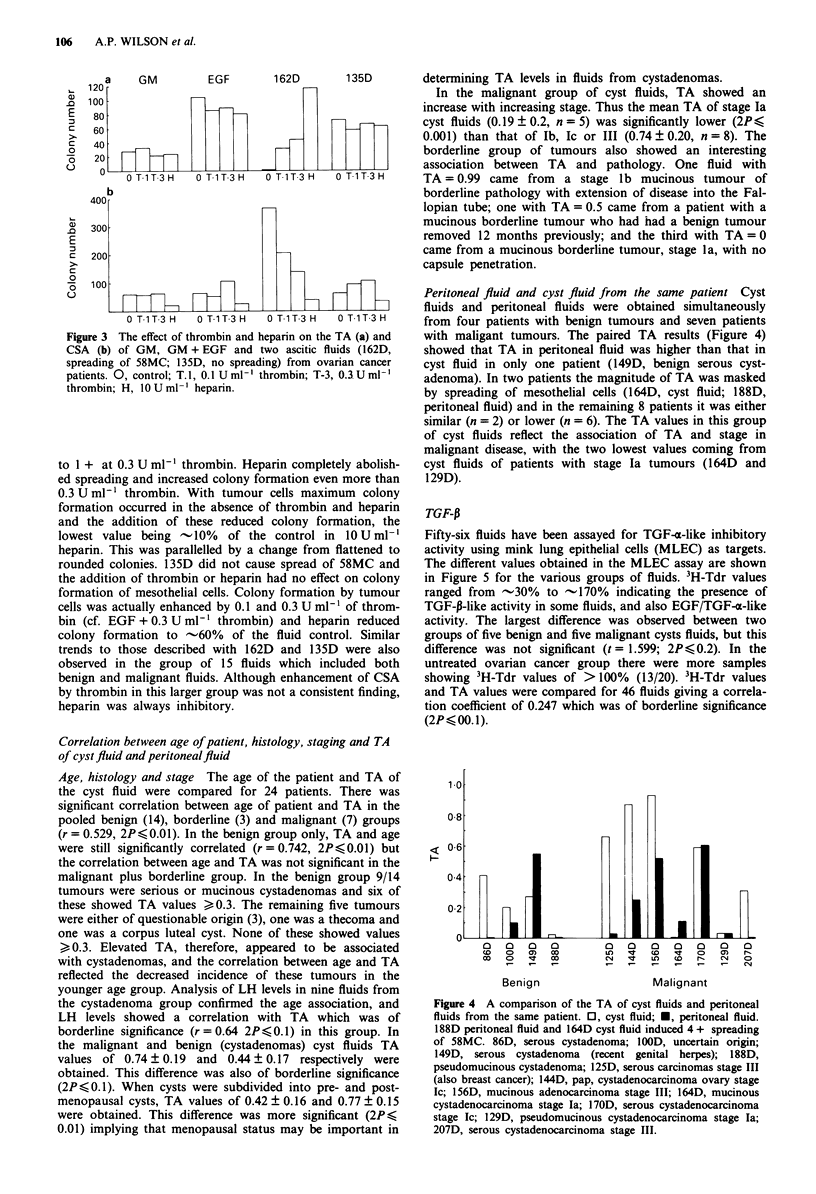
The growth promoting properties of ascitic fluids, cyst fluids and peritoneal fluids from patients with ovarian malignancy, benign ovarian tumours and non-tumour related gynaecological conditions have been investigated using an ovarian carcinoma cell line (OAW 42), mesothelial cells (58MC) and rat kidney cells (NRK-49F). Colony stimulating activity (CSA) for tumour cells and transforming activity (TA) for mesothelial cells were weakly correlated, but whereas elevated TA was tumour-associated, CSA was not. However, TA was not cancer-associated and, although the difference between the mean TA values of benign and malignant cyst fluids was of borderline significance, some benign cyst fluids from cystadenomas showed high TA values. Higher levels of TA in the cystadenomas showed a significant correlation with the menopausal status of the patient and higher levels of TA in the malignant cyst fluid/peritoneal fluid groups were associated with more advanced disease. Results indicated that some fluids contained TGF-beta-like activity, but there was no direct evidence for the presence of TGF-alpha/EGF-like activity in the fluids. Heparin inhibited clonogenic growth of tumour cells but not mesothelial cells. The reduced CSA which was observed after treatment of fluids with both heparin and thrombin implicated coagulation factors in the manifestation of CSA. It was concluded that CSA in the fluids was due, at least partly, to fibrin coagulation, and TA was due to unknown growth factor(s) which may include TGF-beta-like activity. The results are discussed in the context of the aetiology of ovarian carcinoma, and the possible clinical significance of TA.
Full text
PDF

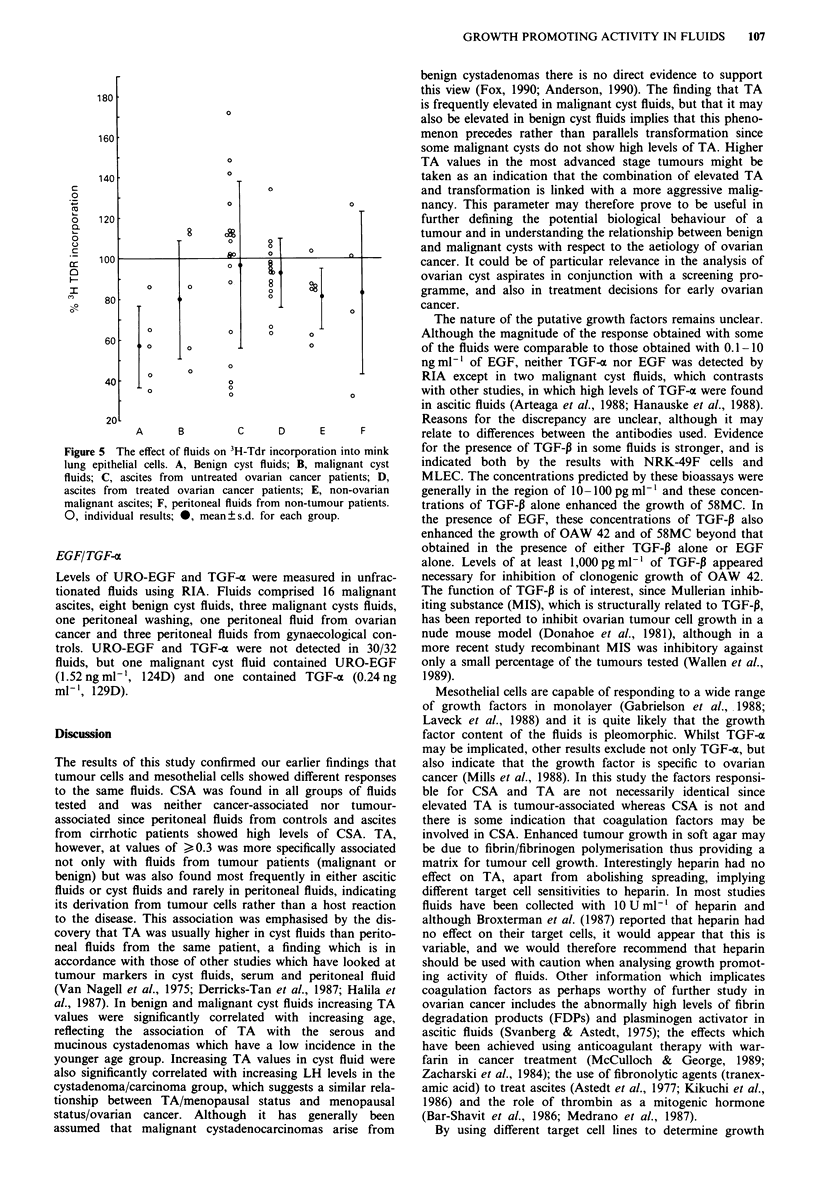

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzano M. A., Roberts A. B., Meyers C. A., Komoriya A., Lamb L. C., Smith J. M., Sporn M. B. Synergistic interaction of two classes of transforming growth factors from murine sarcoma cells. Cancer Res. 1982 Nov;42(11):4776–4778. [PubMed] [Google Scholar]
- Arteaga C. L., Hanauske A. R., Clark G. M., Osborne C. K., Hazarika P., Pardue R. L., Tio F., Von Hoff D. D. Immunoreactive alpha transforming growth factor activity in effusions from cancer patients as a marker of tumor burden and patient prognosis. Cancer Res. 1988 Sep 1;48(17):5023–5028. [PubMed] [Google Scholar]
- Astedt B., Glifberg I., Mattsson W., Tropé C. Arrest of growth of ovarian tumor by tranexamic acid. JAMA. 1977 Jul 11;238(2):154–155. doi: 10.1001/jama.238.2.154. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit R., Hruska K. A., Kahn A. J., Wilner G. D. Hormone-like activity of human thrombin. Ann N Y Acad Sci. 1986;485:335–348. doi: 10.1111/j.1749-6632.1986.tb34595.x. [DOI] [PubMed] [Google Scholar]
- Broxterman H. J., Sprenkels-Schotte C., Engelen P., Leyva A., Pinedo H. M. Analysis of human ascites effect on clonogenic growth of human tumor cell lines and NRK-49F cells in soft agar. Int J Cell Cloning. 1987 Mar;5(2):158–169. doi: 10.1002/stem.5530050208. [DOI] [PubMed] [Google Scholar]
- Donahoe P. K., Fuller A. F., Jr, Scully R. E., Guy S. R., Budzik G. P. Mullerian inhibiting substance inhibits growth of a human ovarian cancer in nude mice. Ann Surg. 1981 Oct;194(4):472–480. doi: 10.1097/00000658-198110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Gregory H., Morris W. P. Acceleration of wound healing by recombinant human urogastrone (epidermal growth factor). J Lab Clin Med. 1986 Aug;108(2):103–108. [PubMed] [Google Scholar]
- Gabrielson E. W., Gerwin B. I., Harris C. C., Roberts A. B., Sporn M. B., Lechner J. F. Stimulation of DNA synthesis in cultured primary human mesothelial cells by specific growth factors. FASEB J. 1988 Aug;2(11):2717–2721. doi: 10.1096/fasebj.2.11.3260881. [DOI] [PubMed] [Google Scholar]
- Gregory H., Thomas C. E., Willshire I. R., Young J. A., Anderson H., Baildam A., Howell A. Epidermal and transforming growth factor alpha in patients with breast tumours. Br J Cancer. 1989 Apr;59(4):605–609. doi: 10.1038/bjc.1989.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory H., Thomas C. E., Young J. A., Willshire I. R., Garner A. The contribution of the C-terminal undecapeptide sequence of urogastrone-epidermal growth factor to its biological action. Regul Pept. 1988 Aug;22(3):217–226. doi: 10.1016/0167-0115(88)90034-1. [DOI] [PubMed] [Google Scholar]
- Halila H., Huhtala M. L., Haglund C., Nordling S., Stenman U. H. Tumour-associated trypsin inhibitor (TATI) in human ovarian cyst fluid. Comparison with CA 125 and CEA. Br J Cancer. 1987 Aug;56(2):153–156. doi: 10.1038/bjc.1987.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanauske A. R., Arteaga C. L., Clark G. M., Buchok J., Marshall M., Hazarika P., Pardue R. L., Von Hoff D. D. Determination of transforming growth factor activity in effusions from cancer patients. Cancer. 1988 May 1;61(9):1832–1837. doi: 10.1002/1097-0142(19880501)61:9<1832::aid-cncr2820610919>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Armour R., Baldwin J. H., Greenfield S. Activity of a kidney epithelial cell growth inhibitor on lung and mammary cells. Cell Biol Int Rep. 1983 Feb;7(2):141–147. doi: 10.1016/0309-1651(83)90027-9. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Kizawa I., Oomori K., Matsuda M., Kato K. Adjuvant effects of tranexamic acid to chemotherapy in ovarian cancer patients with large amount of ascites. Acta Obstet Gynecol Scand. 1986;65(5):453–456. doi: 10.3109/00016348609157383. [DOI] [PubMed] [Google Scholar]
- La Rocca P. J., Rheinwald J. G. Anchorage-independent growth of normal human mesothelial cells: a sensitive bioassay for EGF which discloses the absence of this factor in fetal calf serum. In Vitro Cell Dev Biol. 1985 Jan;21(1):67–72. doi: 10.1007/BF02620917. [DOI] [PubMed] [Google Scholar]
- Laveck M. A., Somers A. N., Moore L. L., Gerwin B. I., Lechner J. F. Dissimilar peptide growth factors can induce normal human mesothelial cell multiplication. In Vitro Cell Dev Biol. 1988 Nov;24(11):1077–1084. doi: 10.1007/BF02620808. [DOI] [PubMed] [Google Scholar]
- McCulloch P., George W. D. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumour growth. Br J Cancer. 1989 Feb;59(2):179–183. doi: 10.1038/bjc.1989.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano E. E., Cafferata E. G., Larcher F. Role of thrombin in the proliferative response of T-47D mammary tumor cells. Mitogenic action and pleiotropic modifications induced together with epidermal growth factor and insulin. Exp Cell Res. 1987 Oct;172(2):354–364. doi: 10.1016/0014-4827(87)90393-4. [DOI] [PubMed] [Google Scholar]
- Mills G. B., May C., McGill M., Roifman C. M., Mellors A. A putative new growth factor in ascitic fluid from ovarian cancer patients: identification, characterization, and mechanism of action. Cancer Res. 1988 Mar 1;48(5):1066–1071. [PubMed] [Google Scholar]
- Svanberg L., Astedt B. Coagulative and fibrinolytic properties of ascitic fluid associated with ovarian tumors. Cancer. 1975 May;35(5):1382–1387. doi: 10.1002/1097-0142(197505)35:5<1382::aid-cncr2820350522>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Uitendaal M. P., Hubers H. A., McVie J. G., Pinedo H. M. Human tumour clonogenicity in agar is improved by cell-free ascites. Br J Cancer. 1983 Jul;48(1):55–59. doi: 10.1038/bjc.1983.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen J. W., Cate R. L., Kiefer D. M., Riemen M. W., Martinez D., Hoffman R. M., Donahoe P. K., Von Hoff D. D., Pepinsky B., Oliff A. Minimal antiproliferative effect of recombinant müllerian inhibiting substance on gynecological tumor cell lines and tumor explants. Cancer Res. 1989 Apr 15;49(8):2005–2011. [PubMed] [Google Scholar]
- Wilson A. P. Characterization of a cell line derived from the ascites of a patient with papillary serous cystadenocarcinoma of the ovary. J Natl Cancer Inst. 1984 Mar;72(3):513–521. [PubMed] [Google Scholar]
- Wilson A. P. Mesothelial cells stimulate the anchorage-independent growth of human ovarian tumour cells. Br J Cancer. 1989 Jun;59(6):876–882. doi: 10.1038/bjc.1989.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharski L. R., Henderson W. G., Rickles F. R., Forman W. B., Cornell C. J., Jr, Forcier R. J., Edwards R. L., Headley E., Kim S. H., O'Donnell J. F. Effect of warfarin anticoagulation on survival in carcinoma of the lung, colon, head and neck, and prostate. Final report of VA Cooperative Study #75. Cancer. 1984 May 15;53(10):2046–2052. doi: 10.1002/1097-0142(19840515)53:10<2046::aid-cncr2820531007>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- van Nagell J. R., Jr, Pletsch Q. A., Goldenberg D. M. A study of cyst fluid and plasma carcinoembryonic antigen in patients with cystic ovarian neoplasms. Cancer Res. 1975 Jun;35(6):1433–1437. [PubMed] [Google Scholar]