Abstract
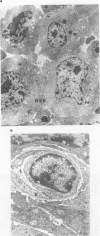
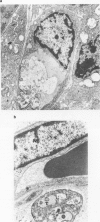
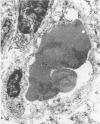
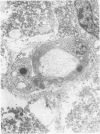
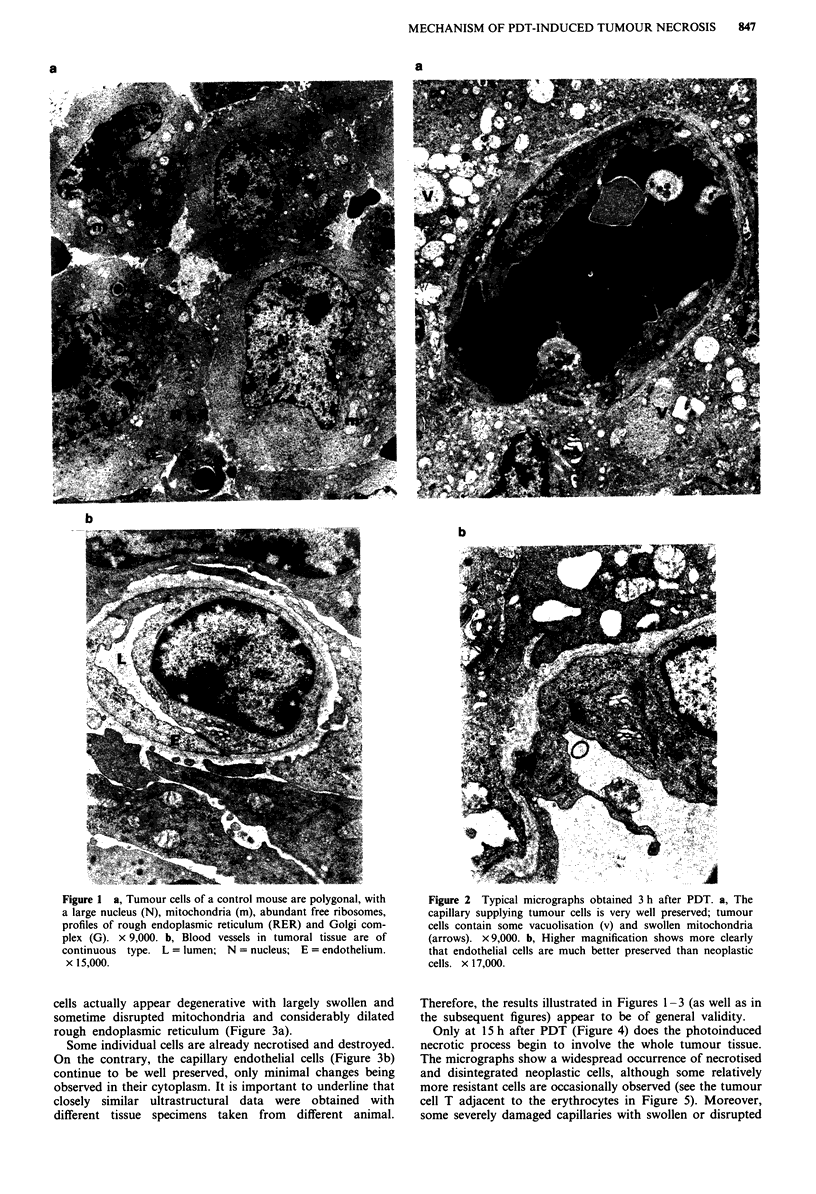
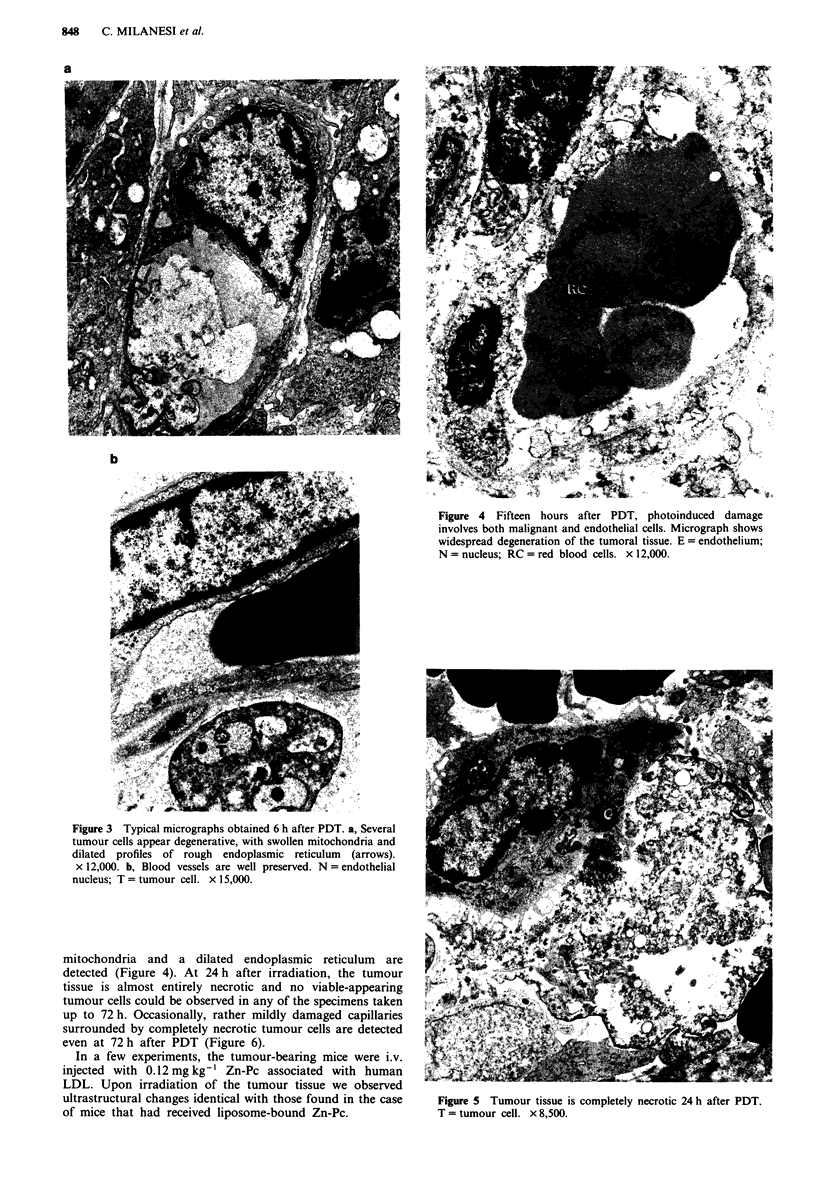
The mechanism of tumour necrosis photosensitised by liposome-delivered Zn(II) phthalocyanine (Zn-Pc) has been studied in mice bearing a transplanted MS-2 fibrosarcoma. Ultrastructural analyses of tumour specimens obtained at different times after red light-irradiation (300 J cm-2, dose-rate 180 mW cm-2) indicate an early (3 h) photodamage of malignant cells especially at the level of the mitochondria and rough endoplasmic reticulum. The cellular damage becomes more evident between 6 h and 15 h after photodynamic therapy. On the other hand, the capillaries supplying the tumour tissue are modified at a much slower rate and appear to be severely damaged only after 15 h from irradiation, when the whole tissue becomes necrotic. Occasionally, mildly damaged capillaries are observed even at 72 h after irradiation. These findings support the hypothesis that low density lipoproteins (LDL) play a major role in the delivery of Zn-Pc to the tumour tissue; the photosensitiser is released specifically to malignant cells as a consequence of a receptor-mediated endocytosis of LDL.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellnier D. A., Lin C. W. Giant cell formation in bladder tumor cells following hematoporphyrin derivative-sensitized photoirradiation. Photochem Photobiol. 1984 Mar;39(3):425–428. doi: 10.1111/j.1751-1097.1984.tb08200.x. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Evolution of the LDL receptor concept-from cultured cells to intact animals. Ann N Y Acad Sci. 1980;348:48–68. doi: 10.1111/j.1749-6632.1980.tb21290.x. [DOI] [PubMed] [Google Scholar]
- Chopp M., Glasberg M. R., Riddle J. M., Hetzel F. W., Welch K. M. Photodynamic therapy of normal cerebral tissue in the cat: a noninvasive model for cerebrovascular thrombosis. Photochem Photobiol. 1987 Jul;46(1):103–108. doi: 10.1111/j.1751-1097.1987.tb04742.x. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J. Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol. 1987 Jun;45(6):879–889. doi: 10.1111/j.1751-1097.1987.tb07898.x. [DOI] [PubMed] [Google Scholar]
- Freitas I. Role of hypoxia in photodynamic therapy of tumors. Tumori. 1985 Jun 30;71(3):251–259. doi: 10.1177/030089168507100306. [DOI] [PubMed] [Google Scholar]
- Gal D., MacDonald P. C., Porter J. C., Simpson E. R. Cholesterol metabolism in cancer cells in monolayer culture. III. Low-density lipoprotein metabolism. Int J Cancer. 1981 Sep 15;28(3):315–319. doi: 10.1002/ijc.2910280310. [DOI] [PubMed] [Google Scholar]
- Henderson B. W., Waldow S. M., Mang T. S., Potter W. R., Malone P. B., Dougherty T. J. Tumor destruction and kinetics of tumor cell death in two experimental mouse tumors following photodynamic therapy. Cancer Res. 1985 Feb;45(2):572–576. [PubMed] [Google Scholar]
- Kessel D., Thompson P., Saatio K., Nantwi K. D. Tumor localization and photosensitization by sulfonated derivatives of tetraphenylporphine. Photochem Photobiol. 1987 Jun;45(6):787–790. doi: 10.1111/j.1751-1097.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- Lee See K., Forbes I. J., Betts W. H. Oxygen dependency of photocytotoxicity with haematoporphyrin derivative. Photochem Photobiol. 1984 May;39(5):631–634. doi: 10.1111/j.1751-1097.1984.tb03902.x. [DOI] [PubMed] [Google Scholar]
- Milanesi C., Biolo R., Reddi E., Jori G. Ultrastructural studies on the mechanism of the photodynamic therapy of tumors. Photochem Photobiol. 1987 Nov;46(5):675–681. doi: 10.1111/j.1751-1097.1987.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Selman S. H., Kreimer-Birnbaum M., Chaudhuri K., Garbo G. M., Seaman D. A., Keck R. W., Ben-Hur E., Rosenthal I. Photodynamic treatment of transplantable bladder tumors in rodents after pretreatment with chloroaluminum tetrasulfophthalocyanine. J Urol. 1986 Jul;136(1):141–145. doi: 10.1016/s0022-5347(17)44759-8. [DOI] [PubMed] [Google Scholar]
- Star W. M., Marijnissen H. P., van den Berg-Blok A. E., Versteeg J. A., Franken K. A., Reinhold H. S. Destruction of rat mammary tumor and normal tissue microcirculation by hematoporphyrin derivative photoradiation observed in vivo in sandwich observation chambers. Cancer Res. 1986 May;46(5):2532–2540. [PubMed] [Google Scholar]
- Zhou C. N., Milanesi C., Jori G. An ultrastructural comparative evaluation of tumors photosensitized by porphyrins administered in aqueous solution, bound to liposomes or to lipoproteins. Photochem Photobiol. 1988 Oct;48(4):487–492. doi: 10.1111/j.1751-1097.1988.tb02850.x. [DOI] [PubMed] [Google Scholar]