Abstract
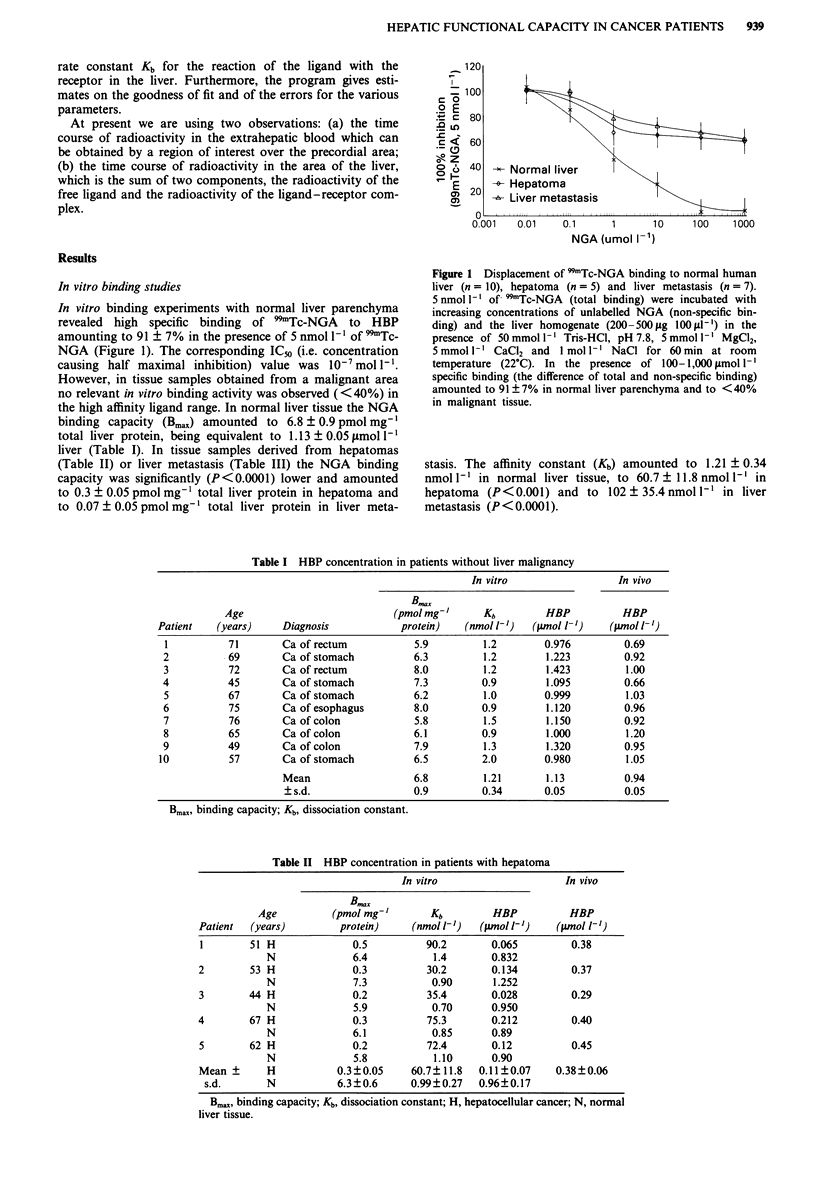
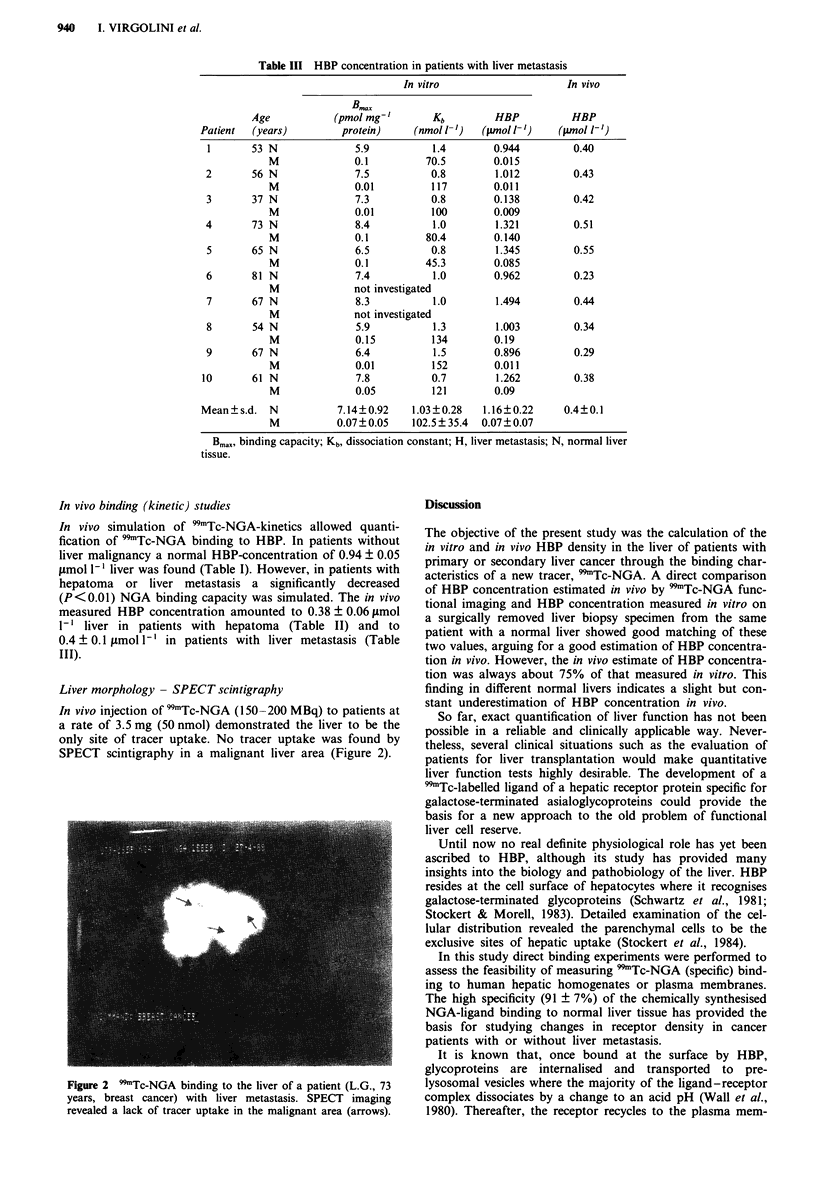
99mTc-galactosylated neoglycoalbumin (99mTc-NGA) is a hepatocyte-specific tracer that, after injection into the blood stream, delivers radioactivity selectively to the liver. This is based upon chemical recognition and binding by the hepatic binding protein (HBP), a receptor specific for galactosylated glycoproteins. Liver tissue samples were obtained intraoperatively from patients undergoing surgery for various cancers. The concentration of specific HBP receptors in the liver (normal liver, hepatoma, liver metastasis) was calculated from the in vitro binding of 99mTc-NGA. One week after surgery, the in vivo HBP density was also measured in some of these patients after injection of 3.5 mg (50 nmol per patient) 99mTc-NGA (150-200 MBq) for simulation of 99mTc-NGA kinetics. Comparison of in vitro and in vivo HBP concentration in the liver showed values in the same concentration range. In patients with hepatoma or liver metastasis a significantly (P less than 0.01) decreased global HBP density was found in vivo compared to controls. The values obtained for in vivo HBP concentration in the liver amounted to 0.38 +/- 0.05 mumol l-1 liver for patients with hepatoma, to 0.4 +/- 0.1 mumol l-1 in patients with liver metastasis and to 94 +/- 0.05 mumol l-1 liver in cancer patients without liver malignancy. In vitro investigation of HBP density revealed the malignant liver tissue to have a significantly (P less than 0.0001) decreased or almost (completely) absent HBP receptor density compared to the normal tissue apart from the cancer area. It is concluded that determination of HBP density in vivo via a specific tracer is a new, simple and reliable approach for the determination of remaining hepatic function in patients with primary or secondary liver cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen P. B., Ranek L., Statland B. E., Tygstrup N. Clearance of antipyrine-dependence of quantitative liver function. Eur J Clin Invest. 1974 Apr;4(2):129–134. doi: 10.1111/j.1365-2362.1974.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Bircher J., Küpfer A., Gikalov I., Preisig R. Aminopyrine demethylation measured by breath analysis in cirrhosis. Clin Pharmacol Ther. 1976 Oct;20(4):484–492. doi: 10.1002/cpt1976204484. [DOI] [PubMed] [Google Scholar]
- Haimes H. B., Stockert R. J., Morell A. G., Novikoff A. B. Carbohydrate-specified endocytosis: localization of ligand in the lysosomal compartment. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6936–6939. doi: 10.1073/pnas.78.11.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcki W., Bircher J., Preisig R. A new look at the plasma disappearance of sulfobromophthalein (BSP): correlation with the BSP transport maximum and the hepatic plasma flow in man. J Lab Clin Med. 1976 Dec;88(6):1019–1031. [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Sawamura T., Kawasato S., Shiozaki Y., Sameshima Y., Nakada H., Tashiro Y. Decrease of a hepatic binding protein specific for asialoglycoproteins with accumulation of serum asialoglycoproteins in galactosamine-treated rats. Gastroenterology. 1981 Sep;81(3):527–533. [PubMed] [Google Scholar]
- Schwartz A. L., Marshak-Rothstein A., Rup D., Lodish H. F. Identification and quantification of the rat hepatocyte asialoglycoprotein receptor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3348–3352. doi: 10.1073/pnas.78.6.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadalnik R. C., Vera D. R., Woodle E. S., Trudeau W. L., Porter B. A., Ward R. E., Krohn K. A., O'Grady L. F. Technetium-99m NGA functional hepatic imaging: preliminary clinical experience. J Nucl Med. 1985 Nov;26(11):1233–1242. [PubMed] [Google Scholar]
- Stockert R. J., Becker F. F. Diminished hepatic binding protein for desialylated glycoproteins during chemical hepatocarcinogenesis. Cancer Res. 1980 Oct;40(10):3632–3634. [PubMed] [Google Scholar]
- Stockert R. J., Haimes H. B., Morell A. G., Novikoff P. M., Novikoff A. B., Quintana N., Sternlieb I. Endocytosis of asialoglycoprotein-enzyme conjugates by hepatocytes. Lab Invest. 1980 Dec;43(6):556–563. [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G. Hepatic binding protein: the galactose-specific receptor of mammalian hepatocytes. Hepatology. 1983 Sep-Oct;3(5):750–757. doi: 10.1002/hep.1840030520. [DOI] [PubMed] [Google Scholar]
- Vera D. R., Krohn K. A., Scheibe P. O., Stadalnik R. C. Identifiability analysis of an in vivo receptor-binding radiopharmacokinetic system. IEEE Trans Biomed Eng. 1985 May;32(5):312–322. doi: 10.1109/TBME.1985.325544. [DOI] [PubMed] [Google Scholar]
- Vera D. R., Krohn K. A., Stadalnik R. C., Scheibe P. O. Tc-99m galactosyl-neoglycoalbumin: in vitro characterization of receptor-mediated binding. J Nucl Med. 1984 Jul;25(7):779–787. [PubMed] [Google Scholar]
- Vera D. R., Krohn K. A., Stadalnik R. C., Scheibe P. O. Tc-99m-galactosyl-neoglycoalbumin: in vivo characterization of receptor-mediated binding to hepatocytes. Radiology. 1984 Apr;151(1):191–196. doi: 10.1148/radiology.151.1.6701314. [DOI] [PubMed] [Google Scholar]
- Vera D. R., Stadalnik R. C., Krohn K. A. Technetium-99m galactosyl-neoglycoalbumin: preparation and preclinical studies. J Nucl Med. 1985 Oct;26(10):1157–1167. [PubMed] [Google Scholar]
- Virgolini I., Angelberger P., Müller C., O'Grady J., Sinzinger H. 99mTc-neoglycoalbumin (NGA)-binding to human hepatic binding protein (HBP) in vitro. Br J Clin Pharmacol. 1990 Feb;29(2):207–214. doi: 10.1111/j.1365-2125.1990.tb03621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini I., Hermann M., Sinzinger H. Decrease of prostaglandin I2 binding sites in thyroid cancer. Br J Cancer. 1988 Nov;58(5):584–588. doi: 10.1038/bjc.1988.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgolini I., Sinzinger H., Müller C., Hermann M. Human hepatocellular cancers show decreased prostaglandin E1 binding capacity. Br J Cancer. 1989 Mar;59(3):407–409. doi: 10.1038/bjc.1989.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall D. A., Wilson G., Hubbard A. L. The galactose-specific recognition system of mammalian liver: the route of ligand internalization in rat hepatocytes. Cell. 1980 Aug;21(1):79–93. doi: 10.1016/0092-8674(80)90116-6. [DOI] [PubMed] [Google Scholar]