Abstract
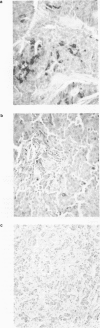
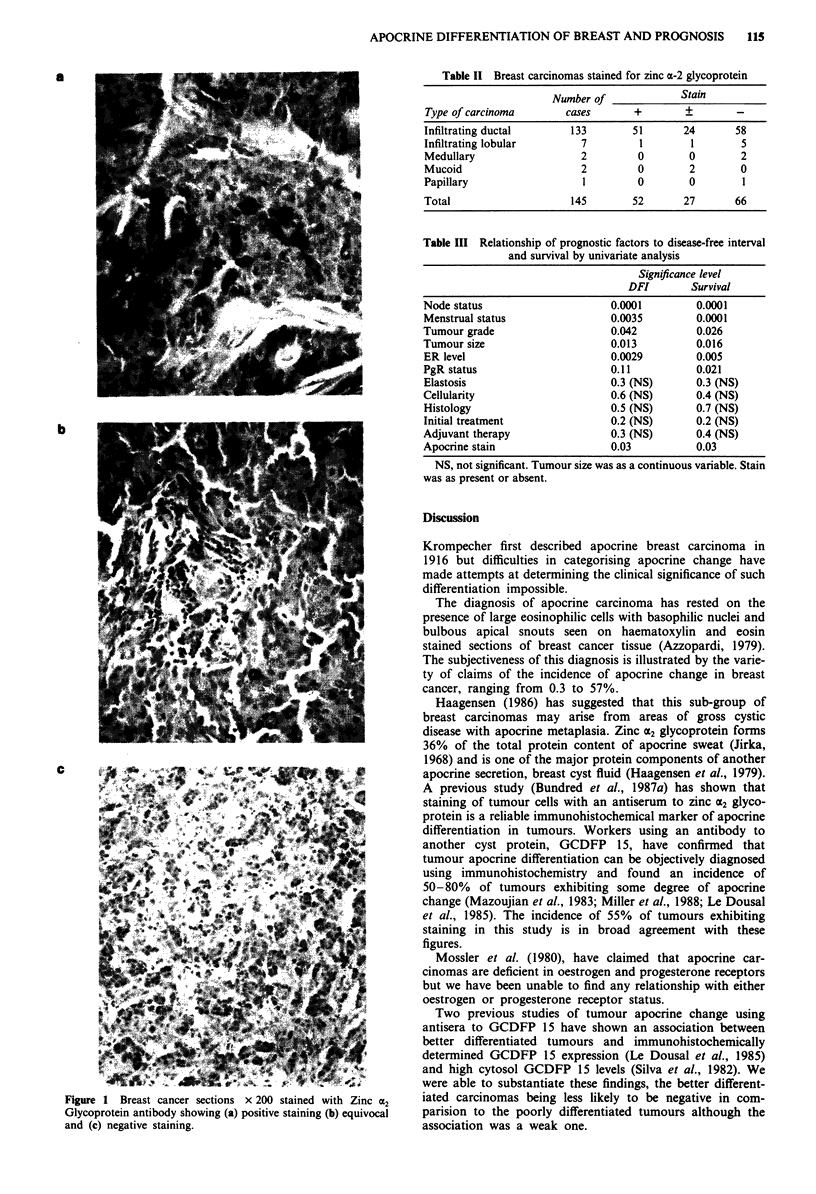
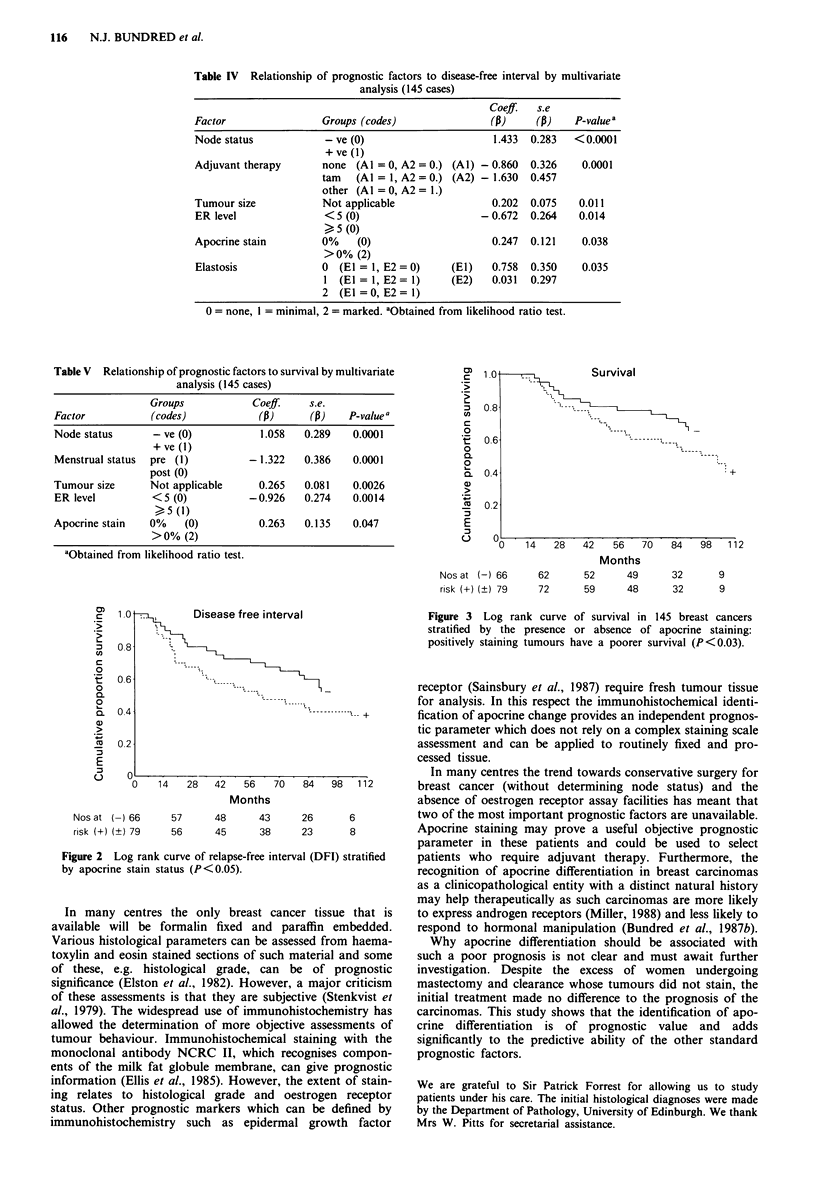
Apocrine differentiation in human breast cancers has been assessed using immunohistochemistry to detect zinc alpha 2 glycoprotein and the findings related to standard prognostic factors, disease free interval (DFI) and survival in 145 women with early breast cancer. Breast tumour samples from women with a minimum follow-up of 5 years were assessed. Routinely fixed and processed tissue was used throughout. Sixty-six (45%) tumours did not stain with the antibody. Fifty-two (36%) exhibited positive apocrine staining while for 27 (19%) only a few cells were reactive. The presence of apocrine differentiation was unrelated to lymph node status, menstrual status, tumour grade or size, oestrogen receptor (E2R) or progesterone receptor status. However, patients whose tumours exhibited apocrine staining had a shorter disease-free interval (DFI) (P = 0.03) and survival (P = 0.03). A Cox's multiple regression analysis of the data found that the presence of staining added significantly (P = 0.047) to the predictive value of node status (P = 0.0001), menstrual status (P = 0.0001), tumour size (P = 0.0026) and E2R status (P = 0.0014) for patient survival. The other seven prognostic factors tested did not reach significance and were rejected from the model. Apocrine differentiation in breast cancer appears to be an independent predictor of poor prognosis tumours.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bundred N. J., Miller W. R., Walker R. A. An immunohistochemical study of the tissue distribution of the breast cyst fluid protein, zinc alpha 2 glycoprotein. Histopathology. 1987 Jun;11(6):603–610. doi: 10.1111/j.1365-2559.1987.tb02670.x. [DOI] [PubMed] [Google Scholar]
- Ellis I. O., Hinton C. P., MacNay J., Elston C. W., Robins A., Owainati A. A., Blamey R. W., Baldwin R. W., Ferry B. Immunocytochemical staining of breast carcinoma with the monoclonal antibody NCRC 11: a new prognostic indicator. Br Med J (Clin Res Ed) 1985 Mar 23;290(6472):881–883. doi: 10.1136/bmj.290.6472.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston C. W., Gresham G. A., Rao G. S., Zebro T., Haybittle J. L., Houghton J., Kearney G. The cancer research campaign (King's/Cambridge trial for early breast cancer: clinico-pathological aspects. Br J Cancer. 1982 May;45(5):655–669. doi: 10.1038/bjc.1982.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E. R., Gregorio R. M., Fisher B., Redmond C., Vellios F., Sommers S. C. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4). Cancer. 1975 Jul;36(1):1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Haagensen D. E., Jr, Mazoujian G., Dilley W. G., Pedersen C. E., Kister S. J., Wells S. A., Jr Breast gross cystic disease fluid analysis. I. Isolation and radioimmunoassay for a major component protein. J Natl Cancer Inst. 1979 Feb;62(2):239–247. [PubMed] [Google Scholar]
- Hawkins R. A., White G., Bundred N. J., Dixon J. M., Miller W. R., Stewart H. J., Forrest A. P. Prognostic significance of oestrogen and progestogen receptor activities in breast cancer. Br J Surg. 1987 Nov;74(11):1009–1013. doi: 10.1002/bjs.1800741118. [DOI] [PubMed] [Google Scholar]
- Jirka M. An alpha(2)-globulin component present in sweat, saliva, tears, human milk, colostrum and cerumen. FEBS Lett. 1968 Jul;1(1):77–80. doi: 10.1016/0014-5793(68)80023-7. [DOI] [PubMed] [Google Scholar]
- Le Doussal V., Zangerle P. F., Collette J., Spyratos F., Hacene K., Briere M., Franchimont P., Gest J. Immunohistochemistry of a component protein of the breast cystic disease fluid with mol. wt 15,000. Eur J Cancer Clin Oncol. 1985 Jun;21(6):715–725. doi: 10.1016/0277-5379(85)90269-x. [DOI] [PubMed] [Google Scholar]
- Mazoujian G., Pinkus G. S., Davis S., Haagensen D. E., Jr Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast. A marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol. 1983 Feb;110(2):105–112. [PMC free article] [PubMed] [Google Scholar]
- Miller W. R., Shivas A. A., Franchimont P., Haagensen D. E. Breast gross cystic disease protein 15 in human breast cancer in culture. Eur J Cancer Clin Oncol. 1988 Feb;24(2):223–228. doi: 10.1016/0277-5379(88)90257-x. [DOI] [PubMed] [Google Scholar]
- Mossler J. A., Barton T. K., Brinkhous A. D., McCarty K. S., Moylan J. A., McCarty K. S., Jr Apocrine differentiation in human mammary carcinoma. Cancer. 1980 Dec 1;46(11):2463–2471. doi: 10.1002/1097-0142(19801201)46:11<2463::aid-cncr2820461127>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Sainsbury J. R., Farndon J. R., Needham G. K., Malcolm A. J., Harris A. L. Epidermal-growth-factor receptor status as predictor of early recurrence of and death from breast cancer. Lancet. 1987 Jun 20;1(8547):1398–1402. doi: 10.1016/s0140-6736(87)90593-9. [DOI] [PubMed] [Google Scholar]
- Silva J. S., Cox C. E., Wells S. A., Jr, Paull D., Dilley W. G., McCarty K. S., Sr, Fetter B. F., Glaubitz L. C., McCarty K. S., Jr Biochemical correlates of morphologic differentiation in human breast cancer. Surgery. 1982 Sep;92(3):443–449. [PubMed] [Google Scholar]
- Stenkvist B., Westman-Naeser S., Vegelius J., Holmquist J., Nordin B., Bengtsson E., Eriksson O. Analysis of reproducibility of subjective grading systems for breast carcinoma. J Clin Pathol. 1979 Oct;32(10):979–985. doi: 10.1136/jcp.32.10.979. [DOI] [PMC free article] [PubMed] [Google Scholar]