Abstract
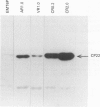
The relationships between resistance to adriamycin, vincristine, colchicine and etopside, expression of P-glycoprotein and CP22 (sorcin), and resistance modification by verapamil and cyclosporin A have been studied in a panel of multidrug-resistant (MDR) mouse tumour cell lines. Whereas there was a generally good correlation between the degree of resistance and the amount of P-glycoprotein, no relationship between resistance and CP22 expression was seen. At 3.3 microM verapamil, the sensitisation of the MDR cell lines was no greater than that of the parent line. At 6.6 microM verapamil, however, sensitisation of the MDR lines generally exceeded that of the parent line, although the line CR 2.0, expressing very high levels of P-glycoprotein was an exception. Little sensitisation to etoposide was seen in any of the lines. When cyclosporin A was used as the sensitiser at either 2.1 or 4.2 microM, there was a greater effect in lines expressing moderate to high levels of P-glycoprotein than in the parent line, although this tendency was less for adriamycin than for the other cytotoxics. Sensitisation to etoposide was much greater with cyclosporin A than with verapamil. At low levels (less than 1 microM) of CsA, however, sensitisation to colchicine was greater in the parent line than in cell line CR 2.0. These studies indicate that chemosensitisation by verapamil and cyclosporin A is extremely complex, depending upon sensitiser dose, the particular cytotoxic and the cell line. At low doses of the sensitisers, the sensitisation may be greater in lines expressing low levels of P-glycoprotein than in lines showing high levels.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck W. T., Cirtain M. C., Danks M. K., Felsted R. L., Safa A. R., Wolverton J. S., Suttle D. P., Trent J. M. Pharmacological, molecular, and cytogenetic analysis of "atypical" multidrug-resistant human leukemic cells. Cancer Res. 1987 Oct 15;47(20):5455–5460. [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Slovak M. L. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989 Jan;59(1):42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley H. M., Twentyman P. R., Workman P. Identification of anthracyclines and related agents that retain preferential activity over adriamycin in multidrug-resistant cell lines, and further resistance modification by verapamil and cyclosporin A. Cancer Chemother Pharmacol. 1989;24(5):284–290. doi: 10.1007/BF00304759. [DOI] [PubMed] [Google Scholar]
- Coley H. M., Twentyman P. R., Workman P. Improved cellular accumulation is characteristic of anthracyclines which retain high activity in multidrug resistant cell lines, alone or in combination with verapamil or cyclosporin A. Biochem Pharmacol. 1989 Dec 15;38(24):4467–4475. doi: 10.1016/0006-2952(89)90658-8. [DOI] [PubMed] [Google Scholar]
- Cornwell M. M., Pastan I., Gottesman M. M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem. 1987 Feb 15;262(5):2166–2170. [PubMed] [Google Scholar]
- Croop J. M., Guild B. C., Gros P., Housman D. E. Genetics of multidrug resistance: relationship of a cloned gene to the complete multidrug resistant phenotype. Cancer Res. 1987 Nov 15;47(22):5982–5988. [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Debenham P. G., Kartner N., Siminovitch L., Riordan J. R., Ling V. DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982 Aug;2(8):881–889. doi: 10.1128/mcb.2.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba M., Kobayashi H., Sakurai Y., Johnson R. K. Active efflux of daunorubicin and adriamycin in sensitive and resistant sublines of P388 leukemia. Cancer Res. 1979 Jun;39(6 Pt 1):2200–2203. [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Koch G., Smith M., Macer D., Webster P., Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986 Dec;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- Koch G., Smith M., Twentyman P., Wright K. Identification of a novel calcium-binding protein (CP22) in multidrug-resistant murine and hamster cells. FEBS Lett. 1986 Jan 20;195(1-2):275–279. doi: 10.1016/0014-5793(86)80176-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Biedler J. L. Increased synthesis of a low molecular weight protein in vincristine-resistant cells. Biochem Biophys Res Commun. 1981 Mar 16;99(1):228–235. doi: 10.1016/0006-291x(81)91736-8. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Naito M., Tsuruo T. Competitive inhibition by verapamil of ATP-dependent high affinity vincristine binding to the plasma membrane of multidrug-resistant K562 cells without calcium ion involvement. Cancer Res. 1989 Mar 15;49(6):1452–1455. [PubMed] [Google Scholar]
- Nooter K., Oostrum R., Jonker R., van Dekken H., Stokdijk W., van den Engh G. Effect of cyclosporin A on daunorubicin accumulation in multidrug-resistant P388 leukemia cells measured by real-time flow cytometry. Cancer Chemother Pharmacol. 1989;23(5):296–300. doi: 10.1007/BF00292407. [DOI] [PubMed] [Google Scholar]
- Ramu A., Spanier R., Rahamimoff H., Fuks Z. Restoration of doxorubicin responsiveness in doxorubicin-resistant P388 murine leukaemia cells. Br J Cancer. 1984 Oct;50(4):501–507. doi: 10.1038/bjc.1984.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. G., Wright K. A., Rabbitts P. H., Twentyman P. R., Koch G. Collateral resistance to verapamil in multidrug-resistant mouse tumor cells. J Natl Cancer Inst. 1989 Oct 18;81(20):1588–1590. doi: 10.1093/jnci/81.20.1588. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979 Dec 25;254(24):12701–12705. [PubMed] [Google Scholar]
- Rockwell S. C., Kallman R. F., Fajardo L. F. Characteristics of a serially transplanted mouse mammary tumor and its tissue-culture-adapted derivative. J Natl Cancer Inst. 1972 Sep;49(3):735–749. [PubMed] [Google Scholar]
- Safa A. R., Glover C. J., Sewell J. L., Meyers M. B., Biedler J. L., Felsted R. L. Identification of the multidrug resistance-related membrane glycoprotein as an acceptor for calcium channel blockers. J Biol Chem. 1987 Jun 5;262(16):7884–7888. [PubMed] [Google Scholar]
- Slater L. M., Sweet P., Stupecky M., Gupta S. Cyclosporin A reverses vincristine and daunorubicin resistance in acute lymphatic leukemia in vitro. J Clin Invest. 1986 Apr;77(4):1405–1408. doi: 10.1172/JCI112450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater L. M., Sweet P., Stupecky M., Wetzel M. W., Gupta S. Cyclosporin A corrects daunorubicin resistance in Ehrlich ascites carcinoma. Br J Cancer. 1986 Aug;54(2):235–238. doi: 10.1038/bjc.1986.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Potentiation of vincristine and Adriamycin effects in human hemopoietic tumor cell lines by calcium antagonists and calmodulin inhibitors. Cancer Res. 1983 May;43(5):2267–2272. [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Bleehen N. M. Drug resistance in human lung cancer cell lines: cross-resistance studies and effects of the calcium transport blocker, verapamil. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1355–1358. doi: 10.1016/0360-3016(86)90170-7. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., White D. J. Cyclosporin A and its analogues as modifiers of adriamycin and vincristine resistance in a multi-drug resistant human lung cancer cell line. Br J Cancer. 1987 Jul;56(1):55–57. doi: 10.1038/bjc.1987.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987 Sep;56(3):279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R. Response to chemotherapy of EMT6 spheroids as measured by growth delay and cell survival. Br J Cancer. 1980 Aug;42(2):297–304. doi: 10.1038/bjc.1980.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Bliek A. M., Van der Velde-Koerts T., Ling V., Borst P. Overexpression and amplification of five genes in a multidrug-resistant Chinese hamster ovary cell line. Mol Cell Biol. 1986 May;6(5):1671–1678. doi: 10.1128/mcb.6.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]