Abstract
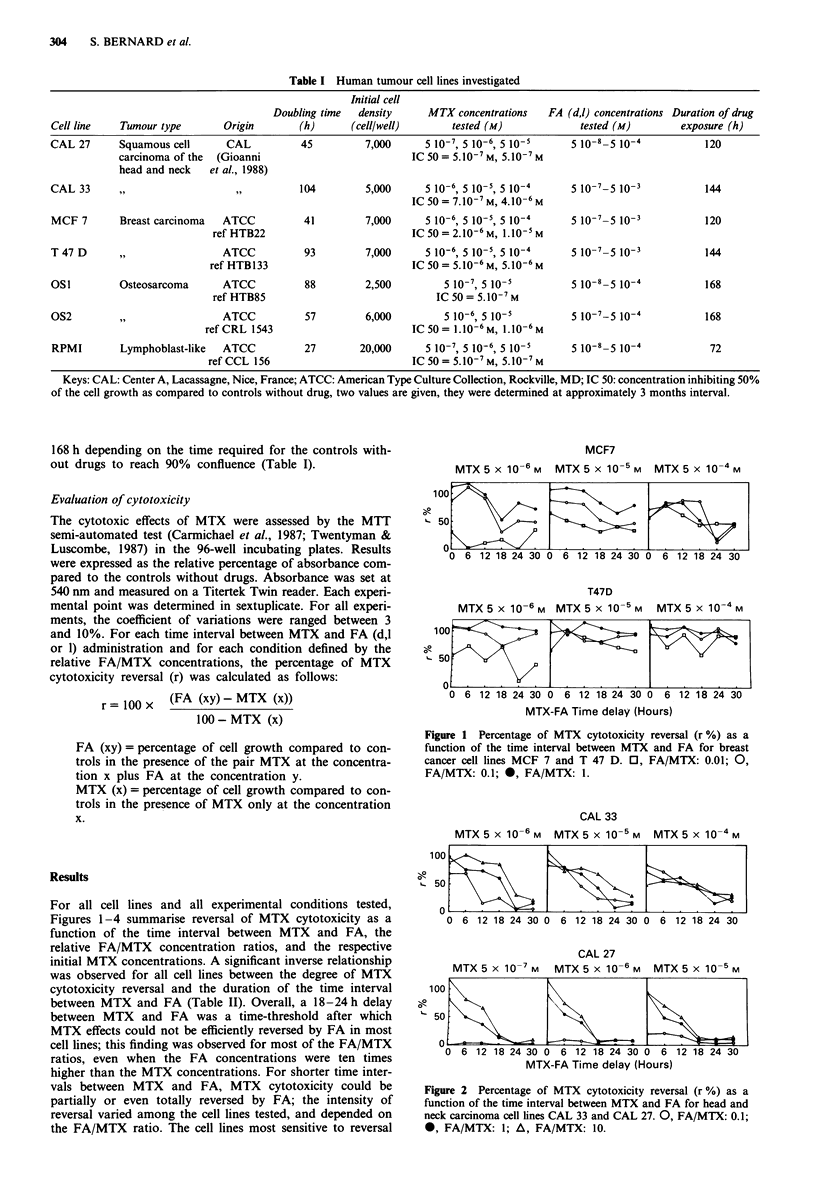
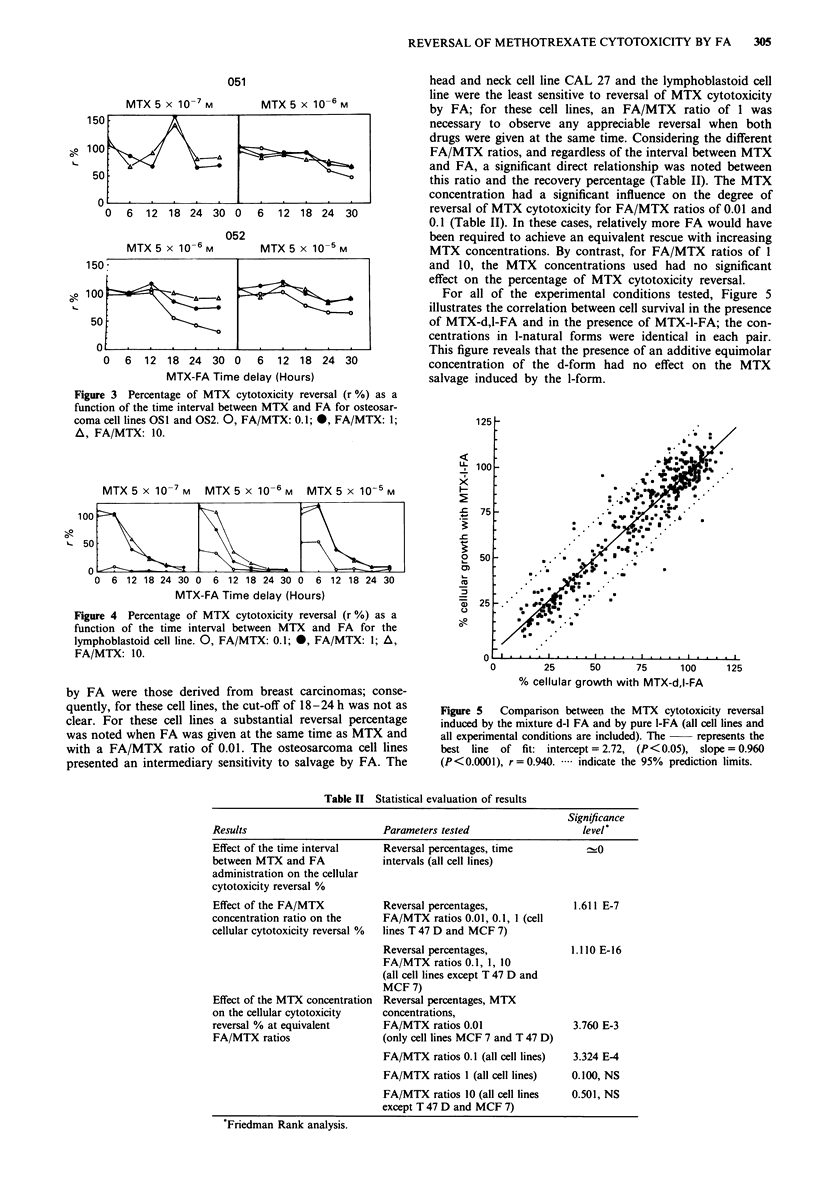
The cytotoxicity of methotrexate (MTX) on representative human tumour cell lines (two cell lines from head and neck carcinomas, two from breast carcinomas, two from osteosarcomas and one lymphoblastoid cell line) was evaluated to: (1) examine the optimal time interval between MTX and folinic acid (FA) administration; (2) determine the critical FA/MTX concentration ratios; and (3) compare the relative effects of the equimolar mixture d,I-FA and I-FA. The cytotoxic effects of MTX were assessed by the MTT semi-automated test. For all of the cell lines tested, a significant inverse relationship was noted between the degree of MTX cytotoxicity reversal and the duration of the time interval between MTX and FA administration. Overall an 18-24 h interval between MTX and FA represented a time-threshold after which MTX effects could not efficiently be reversed by FA in most cell lines. With shorter time intervals between MTX and FA, MTX cytotoxicity could be partially on even totally reversed by FA; the intensity of reversal varied among the cell lines tested, and depended on the FA/MTX ratio. Regardless of the interval between MTX and FA, analysis of the various FA/MTX ratios revealed a significant direct relationship between this ratio and the percentage of recovery. Presence of the d-form had no influence on the MTX rescue capacity of the I-form; this suggests that the presence of the d-FA is unlikely to have any significant clinical consequences.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackland S. P., Schilsky R. L. High-dose methotrexate: a critical reappraisal. J Clin Oncol. 1987 Dec;5(12):2017–2031. doi: 10.1200/JCO.1987.5.12.2017. [DOI] [PubMed] [Google Scholar]
- Bertrand R., Jolivet J. Lack of interference by the unnatural isomer of 5-formyltetrahydrofolate with the effects of the natural isomer in leucovorin preparations. J Natl Cancer Inst. 1989 Aug 2;81(15):1175–1178. doi: 10.1093/jnci/81.15.1175. [DOI] [PubMed] [Google Scholar]
- Borsi J. D., Moe P. J. A comparative study on the pharmacokinetics of methotrexate in a dose range of 0.5 g to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer. 1987 Jul 1;60(1):5–13. doi: 10.1002/1097-0142(19870701)60:1<5::aid-cncr2820600103>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Browman G. P., Goodyear M. D., Levine M. N., Russell R., Archibald S. D., Young J. E. Modulation of the antitumor effect of methotrexate by low-dose leucovorin in squamous cell head and neck cancer: a randomized placebo-controlled clinical trial. J Clin Oncol. 1990 Feb;8(2):203–208. doi: 10.1200/JCO.1990.8.2.203. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Diddens H., Teufel T., Niethammer D. High-dose methotrexate therapy with leucovorin rescue: in vitro investigations on human osteosarcoma cell lines. Cancer Chemother Pharmacol. 1987;20(2):128–132. doi: 10.1007/BF00253966. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Hutson P. R., Stewart C. F., Cairnes D. A., Bowman W. P., Rivera G., Crom W. R. Methotrexate cerebrospinal fluid and serum concentrations after intermediate-dose methotrexate infusion. Clin Pharmacol Ther. 1983 Mar;33(3):301–307. doi: 10.1038/clpt.1983.37. [DOI] [PubMed] [Google Scholar]
- Gioanni J., Fischel J. L., Lambert J. C., Demard F., Mazeau C., Zanghellini E., Ettore F., Formento P., Chauvel P., Lalanne C. M. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: establishment, characterization and response to cytotoxic treatment. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1445–1455. doi: 10.1016/0277-5379(88)90335-5. [DOI] [PubMed] [Google Scholar]
- Jaffe N., Robertson R., Ayala A., Wallace S., Chuang V., Anzai T., Cangir A., Wang Y. M., Chen T. Comparison of intra-arterial cis-diamminedichloroplatinum II with high-dose methotrexate and citrovorum factor rescue in the treatment of primary osteosarcoma. J Clin Oncol. 1985 Aug;3(8):1101–1104. doi: 10.1200/JCO.1985.3.8.1101. [DOI] [PubMed] [Google Scholar]
- Jolivet J., Cowan K. H., Curt G. A., Clendeninn N. J., Chabner B. A. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983 Nov 3;309(18):1094–1104. doi: 10.1056/NEJM198311033091805. [DOI] [PubMed] [Google Scholar]
- Milano G., Thyss A., Renée N., Vallicioni J., Santini J., Schneider M., Demard F. Altered pharmacokinetics and clinical consequences of low dose methotrexate plus cisplatin in the treatment of advanced head and neck cancer. Eur J Cancer Clin Oncol. 1986 Jul;22(7):843–847. doi: 10.1016/0277-5379(86)90372-x. [DOI] [PubMed] [Google Scholar]
- Newman E. M., Straw J. A., Doroshow J. H. Pharmacokinetics of diastereoisomers of (6R,S)-folinic acid (leucovorin) in humans during constant high-dose intravenous infusion. Cancer Res. 1989 Oct 15;49(20):5755–5760. [PubMed] [Google Scholar]
- Pinedo H. M., Zaharko D. S., Bull J. M., Chabner B. A. The reversal of methotrexate cytotoxicity to mouse bone marrow cells by leucovorin and nucleosides. Cancer Res. 1976 Dec;36(12):4418–4424. [PubMed] [Google Scholar]
- Rosen G., Caparros B., Huvos A. G., Kosloff C., Nirenberg A., Cacavio A., Marcove R. C., Lane J. M., Mehta B., Urban C. Preoperative chemotherapy for osteogenic sarcoma: selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982 Mar 15;49(6):1221–1230. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Schilsky R. L., Choi K. E., Vokes E. E., Guaspari A., Guarnieri C., Whaling S., Liebner M. A. Clinical pharmacology of the stereoisomers of leucovorin during repeated oral dosing. Cancer. 1989 Mar 15;63(6 Suppl):1018–1021. doi: 10.1002/1097-0142(19890315)63:6+<1018::aid-cncr2820631305>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schrøder H., Jensen K. B., Brandsborg M. Lack of correlation between methotrexate concentrations in serum, saliva and sweat after 24 h methotrexate infusions. Br J Clin Pharmacol. 1987 Oct;24(4):537–541. doi: 10.1111/j.1365-2125.1987.tb03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Moccio D. M., Dorick D. M. Optimization of high-dose methotrexate with leucovorin rescue therapy in the L1210 leukemia and sarcoma 180 murine tumor models. Cancer Res. 1978 Feb;38(2):345–353. [PubMed] [Google Scholar]
- Stoller R. G., Hande K. R., Jacobs S. A., Rosenberg S. A., Chabner B. A. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977 Sep 22;297(12):630–634. doi: 10.1056/NEJM197709222971203. [DOI] [PubMed] [Google Scholar]
- Stoller R. G., Kaplan H. G., Cummings F. J., Calabresi P. A clinical and pharmacological study of high-dose methotrexate with minimal leucovorin rescue. Cancer Res. 1979 Mar;39(3):908–912. [PubMed] [Google Scholar]
- Straw J. A., Szapary D., Wynn W. T. Pharmacokinetics of the diastereoisomers of leucovorin after intravenous and oral administration to normal subjects. Cancer Res. 1984 Jul;44(7):3114–3119. [PubMed] [Google Scholar]
- Twentyman P. R., Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987 Sep;56(3):279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. D., Nichols A. P., Bender R. A. Membrane transport of methotrexate in human lymphoblastoid cells. Cancer Res. 1978 Mar;38(3):668–671. [PubMed] [Google Scholar]


