Abstract
Thirty-five patients with advanced malignant disease have been treated as outpatients with increasing doses (0.1-100 mcg) of interleukin 2 (IL2) by once daily self-administered subcutaneous (s.c.) injection, 5 days weekly for 8 weeks followed by a 4 week observation period. Systemic side effects were not experienced by patients at the 3 lower doses. Three patients required dose reduction from 100 mcg daily because of intolerance (fever, rash, lethargy, nausea and vomiting) and one patient was discontinued because of dyspnoea. We observed immunological effects at the 100 mcg dose (but not at the lower doses). These consisted of (a) a modest sustained lymphocytosis, (b) eosinophilia in six (out of nine) patients and (c) a significant rise in IL2-stimulated peripheral blood lymphocyte activated killer (LAK) cell activity in six (out of nine) patients to a mean of 2.0 times pretreatment levels (P less than 0.01). Two (out of nine) patients with renal cell carcinoma treated with 100 mcg daily had partial responses of duration 4 and 9 months respectively and a further three had disease stabilisation for at least 3 months. Low dose long-term s.c. IL2 is clinically and immunologically active, and in comparison to other IL2 regimens it has minor toxicity and is easy to administer. These characteristics make low dose s.c. IL2 suitable for study in the adjuvant setting.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. A., Jones S. E., McGuffey P. Phase II trial of outpatient interleukin-2 in malignant lymphoma, chronic lymphocytic leukemia, and selected solid tumors. J Clin Oncol. 1989 Jan;7(1):75–80. doi: 10.1200/JCO.1989.7.1.75. [DOI] [PubMed] [Google Scholar]
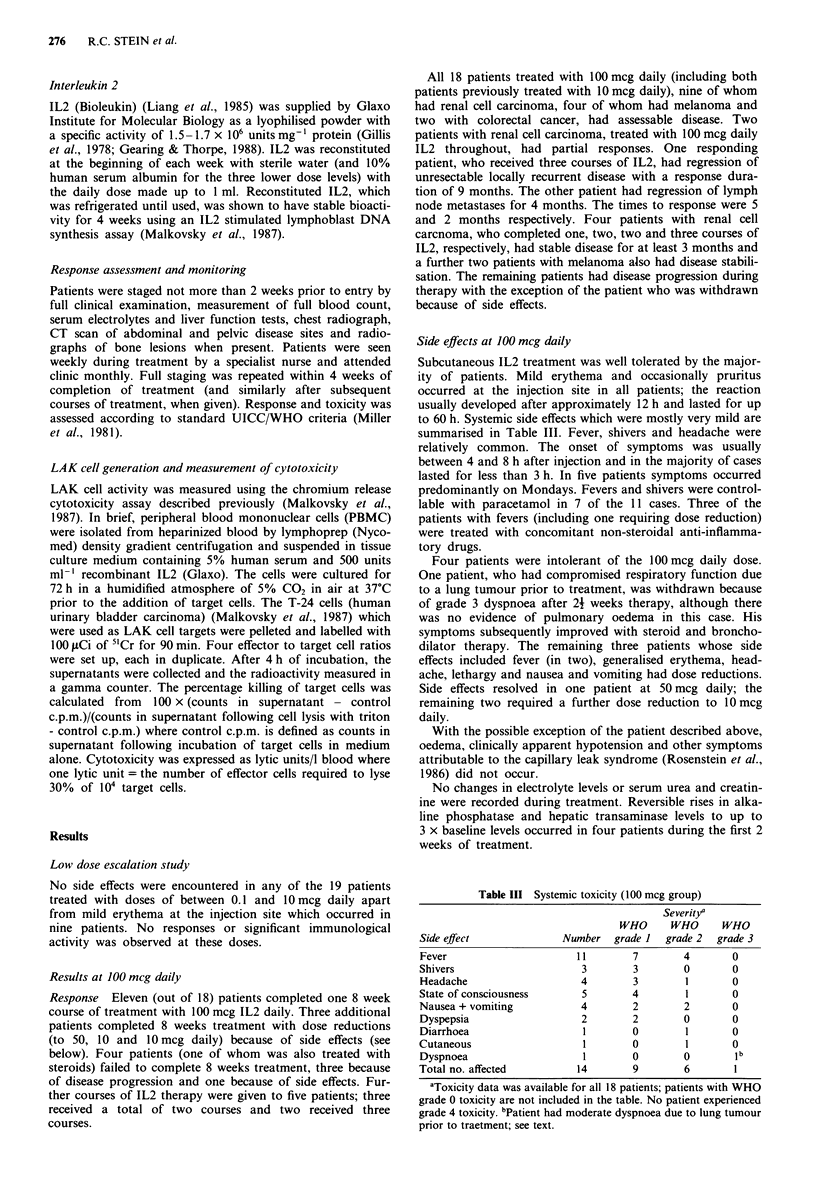
- Boldt D. H., Mills B. J., Gemlo B. T., Holden H., Mier J., Paietta E., McMannis J. D., Escobedo L. V., Sniecinski I., Rayner A. A. Laboratory correlates of adoptive immunotherapy with recombinant interleukin-2 and lymphokine-activated killer cells in humans. Cancer Res. 1988 Aug 1;48(15):4409–4416. [PubMed] [Google Scholar]
- Creekmore S. P., Harris J. E., Ellis T. M., Braun D. P., Cohen I. I., Bhoopalam N., Jassak P. F., Cahill M. A., Canzoneri C. L., Fisher R. I. A phase I clinical trial of recombinant interleukin-2 by periodic 24-hour intravenous infusions. J Clin Oncol. 1989 Feb;7(2):276–284. doi: 10.1200/JCO.1989.7.2.276. [DOI] [PubMed] [Google Scholar]
- Fisher R. I., Coltman C. A., Jr, Doroshow J. H., Rayner A. A., Hawkins M. J., Mier J. W., Wiernik P., McMannis J. D., Weiss G. R., Margolin K. A. Metastatic renal cancer treated with interleukin-2 and lymphokine-activated killer cells. A phase II clinical trial. Ann Intern Med. 1988 Apr;108(4):518–523. doi: 10.7326/0003-4819-108-4-518. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Thorpe R. The international standard for human interleukin-2. Calibration by international collaborative study. J Immunol Methods. 1988 Nov 10;114(1-2):3–9. doi: 10.1016/0022-1759(88)90145-7. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goldstein D., Sosman J. A., Hank J. A., Weil-Hillman G., Moore K. H., Borchert A., Bechhofer R., Storer B., Kohler P. C., Levitt D. Repetitive weekly cycles of interleukin 2: effect of outpatient treatment with a lower dose of interleukin 2 on non-major histocompatibility complex-restricted killer activity. Cancer Res. 1989 Dec 1;49(23):6832–6839. [PubMed] [Google Scholar]
- Liang S. M., Allet B., Rose K., Hirschi M., Liang C. M., Thatcher D. R. Characterization of human interleukin 2 derived from Escherichia coli. Biochem J. 1985 Jul 15;229(2):429–439. doi: 10.1042/bj2290429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Loveland B., North M., Asherson G. L., Gao L., Ward P., Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987 Jan 15;325(6101):262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- Marumo K., Muraki J., Ueno M., Tachibana M., Deguchi N., Baba S., Jitsukawa S., Hata M., Tazaki H. Immunologic study of human recombinant interleukin-2 (low-dose) in patients with advanced renal cell carcinoma. Urology. 1989 Mar;33(3):219–225. doi: 10.1016/0090-4295(89)90396-8. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Oliver R. T., Crosby D., Nouri A., Scott E., Galazka A. Evaluation of the effect of continuous infusion recombinant interleukin-2 (bioleukin) on peripheral blood leucocytes of patients with terminal malignancy. Br J Cancer. 1989 Dec;60(6):934–937. doi: 10.1038/bjc.1989.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenstein M., Ettinghausen S. E., Rosenberg S. A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986 Sep 1;137(5):1735–1742. [PubMed] [Google Scholar]
- Sondel P. M., Kohler P. C., Hank J. A., Moore K. H., Rosenthal N. S., Sosman J. A., Bechhofer R., Storer B. Clinical and immunological effects of recombinant interleukin 2 given by repetitive weekly cycles to patients with cancer. Cancer Res. 1988 May 1;48(9):2561–2567. [PubMed] [Google Scholar]
- Urba W. J., Steis R. G., Longo D. L., Kopp W. C., Maluish A. E., Marcon L., Nelson D. L., Stevenson H. C., Clark J. W. Immunomodulatory properties and toxicity of interleukin 2 in patients with cancer. Cancer Res. 1990 Jan 1;50(1):185–192. [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]


