Abstract
Animal studies and neuropsychological tests of patients with temporal lobe epilepsy have demonstrated the importance of human medial temporal lobes for memory formation. In addition, more recent studies have shown that the human hippocampal region is also involved in novelty detection. However, the exact contribution of the hippocampus proper to these processes is still unknown. To examine further its role we compared event-related potentials recorded within the medial temporal lobes in 29 temporal lobe epilepsy patients with and 21 without hippocampal sclerosis. While in patients with extrahippocampal lesions but without hippocampal sclerosis event-related potentials to first presentations and repetitions of words were reduced on the side of the epileptogenic focus, in patients with hippocampal sclerosis only those to first presentations but not to repetitions were affected. Because sclerosis of the hippocampus proper selectively reduced event-related potentials to new but not old verbal stimuli, it can be concluded that the human hippocampus proper contributes to verbal novelty detection.
The importance of the human hippocampal formation for memory processes has been known for a long time (1–5). Since the early report by Scoville and Milner (6), the hippocampal formation has been suggested to constitute a temporal memory buffer (7, 8), to act as a behavioral inhibition system (9), to be selectively involved in the representation of cognitive maps for spatial memory (10), or to constitute a decisive component of the declarative memory system (11, 12). In addition, several studies demonstrated that the hippocampus proper and adjacent structures including the subiculum, perirhinal, entorhinal, and parahippocampal areas may subserve different aspects of memory processing (5, 13–15). Yet it cannot be excluded that the declarative memory function is based on all structures of the hippocampal system, and the degree of memory impairment depends on the degree of damage to the system as a whole (16). Thus, controversies remain about the exact contributions of the hippocampus to memory formation. Although it has been proposed that the hippocampus in contrast to the parahippocampal region mediates an organization of memories according to relevant relationships among items (17), there are animal studies in which hippocampal lesions did not interfere with performance in tasks requiring the learning of stimulus–stimulus associations (18–20). The conditional discriminations involved in these tasks do not seem to depend on novelty detection, which on the other hand may be an important prerequisite for encoding and storing of information. Converging evidence from single neuron recordings (21–23), positron-emission tomography studies (24), functional magnetic resonance imaging (25), and electrophysiological studies with surface electrodes (26) support the hypothesis that the hippocampal region is critically involved in novelty detection, but it is unclear if the hippocampus proper itself contributes to this task.
Event-related potentials (ERPs) recorded from the scalp in word-recognition tasks have been shown to be sensitive to medial temporal lobe lesions: differences between ERPs to “old” and “new” items that can be seen in healthy subjects were diminished in patients who had undergone anterior temporal lobectomy (27, 28). Recording intracranial event-related potentials from depth electrodes in patients with pharmacoresistent temporal lobe epilepsy (TLE) can help to identify hippocampal contributions to memory processes because electrodes are placed within the hippocampal region, and recordings of high spatial resolution from patients with atrophy and sclerosis of the hippocampus proper can be compared with those from patients with extrahippocampal lesions but nonsclerotic hippocampi. In addition, the high signal-to-noise ratio of medial temporal recordings allows the correlation of event-related potentials in memory paradigms with the graded memory deficits of TLE patients. Several studies have found event-related potentials elicited by words in the anterior medial temporal lobes (AMTL-N400) (29–35). Upon repetitions these were reduced in amplitude. This new-minus-old repetition effect indicates that the hippocampal formation participates in verbal memory processes, as does the finding that AMTL-N400s to first presentations of words recorded in the dominant hemisphere are correlated with verbal recall performance (36). However, it still remains unclear if and how the hippocampus proper contributes to the processing of words in recognition memory tasks. To investigate this question we compared AMTL-N400s to words in TLE patients with hippocampal sclerosis to those in patients with extrahippocampal epileptogenic lesions of the temporal lobe but nonsclerotic hippocampi. If the hippocampus proper contributes in a specific way to word processing in recognition memory tasks, there should be different alterations of AMTL-N400s in both groups as a result of the different epileptogenic lesions. Otherwise, it could be concluded that the medial temporal lobe as a whole participates synergistically in this neuropsychological task. Furthermore, if the hippocampus proper either detects the novelty of words presented for the first time, or contributes to the recognition of word repetitions, then either AMTL-N400s to first presentations or to repetitions should be affected specifically by hippocampal sclerosis.
METHODS
Subjects.
Intracranial ERPs are a very sensitive method for identifying the epileptogenic temporal lobe in patients with medically intractable TLE. This method is used by our group in addition to the invasive recording of seizures when localization of the primary epileptogenic area cannot be identified by noninvasive methods. The patients in the current study had depth electrodes implanted in the medial temporal lobes as part of this presurgical evaluation for resective surgery. Of the 79 available patients, 11 patients were not considered for this study because spike activity interfered with the ERP recordings. Furthermore, given that the analysis of effects of epileptogenic temporal lobe lesions on medial temporal ERPs depends at least in part on comparisons with ERPs from the unaffected temporal lobe, 12 of the remaining 68 patients were excluded who were not seizure free postoperatively. An additional 6 patients who identified less than 50 word repetitions also were excluded. Thus 50 patients (20 females; age range 13–51; 27 left, 23 right TLE) were included in the study. Informed consent was obtained from all patients. Presurgical findings suggested hippocampal sclerosis in 29 patients. In this group 5 temporal lobectomies and 24 selective amygdalo-hippocampectomies were performed. MRI scans demonstrated extrahippocampal lesions without signs of hippocampal sclerosis in 21 patients in whom 19 extended lesionectomies without hippocampectomy and 2 temporal lobectomies including lesionectomy and hippocampectomy were performed. The exact cortical location of extrahippocampal lesions varied from patient to patient but never included areas critical for language production or perception, as demonstrated by electrostimulation mapping. In both groups, both MRI scans and postoperative seizure freedom suggested no dual pathology, and postoperative histological analyses confirmed the presurgical diagnosis in all patients. Based on these findings it was possible to separate two completely dissociable groups of 29 patients with and 21 without hippocampal sclerosis. Both groups did not differ with regard to intelligence or sustained attention. However, patients with hippocampal sclerosis performed significantly worse in verbal and nonverbal list learning tasks (see Table 1). Details of the neuropsychological test battery have been published elsewhere (37). When ERPs were recorded, blood levels of the anticonvulsant medication were within the so-called “therapeutic range” in all patients. Most of them received a carbamazepine monotherapy with blood levels between 8 and 12 μg/ml.
Table 1.
Test scores for patients with and without hippocampal sclerosis (HS).
| Parameter | IQ | Sustained attention | Immediate verbal recall, % | Delayed verbal recall, % | Nonverbal learning, % |
|---|---|---|---|---|---|
| HS | 101 ± 10 | 105 ± 12 | 66.7 ± 15.7** | 43.8 ± 21.9** | 47.6 ± 31.3* |
| No HS | 103 ± 13 | 103 ± 10 | 82.0 ± 11.2** | 65.1 ± 18.5** | 71.2 ± 21.2* |
The table contains IQ values, standardized values for sustained attention (“letter cancellation test”) and mean proportions correct out of the total possible for memory tests (a modified German version of the Rey Auditory Verbal Learning Test and a figurative list learning task (“Diagnostikum für Cerebralschäden, DCS”).
*P < 0.05.
**P < 0.005 (MANOVA and t tests for paired samples).
Recording Methods and Data Analysis.
We recorded stereo-electroencephalograms from bilateral depth electrodes implanted stereotactically along the longitudinal axis of the hippocampus with the amygdala as target of the most anterior contact. Details of implantation and recording procedures can be found in Grunwald et al. (35) and Elger et al. (36). Electrode placements were verified by postimplant computer tomography and MRI. In a visual word recognition paradigm patients had to indicate whether a word was presented for the first time or if it was a repetition by pressing one of two buttons. Words were presented once every 1,800 ± 200 ms, 75 with a lag of 3 ± 1 intervening items and 75 with a delay of 14 ± 4 intervening stimuli. Data were amplified with a bandpass filter setting of 0.03–85 Hz (12 dB/oct.). After 12 bit A/D conversion signals were written continuously to a hard disk by using a sampling rate of 173 Hz/channel. Selective averaging was performed on 1,200-ms stimulus-related epochs. Epochs were rejected in case of false or missing reactions within a time window of 200–1,500 ms. Eleven of 79 patients were excluded from the study in whom signals were found by visual inspection to be contaminated by epilepsy-specific potentials like spikes or sharp waves. ERP components were identified by visual inspection. Their peak amplitudes were measured relative to the mean amplitude of a 200-ms prestimulus baseline. For grand averages and statistical analyses measurements from the depth electrode contact with the largest negativity from 300 to 600 ms on both sides were selected. The locations of these contacts were determined by visual inspection of MRIs with reference to cross sections published by Duvernoy (38; see Fig. 1A). Amplitude measurements were subjected to MANOVA and post-hoc univariate F tests to test amplitude differences of potentials recorded ipsilateral and contralateral to the epileptogenic focus. Effects of repetition on potentials elicited by words were tested by repeated measures ANOVA (F test with Greenhouse–Geisser corrections for P values). In addition, performance scores in the word-recognition paradigm were correlated with left and right AMTL-N400s to first presentations and repetitions as well as with new-minus-old repetition effects.
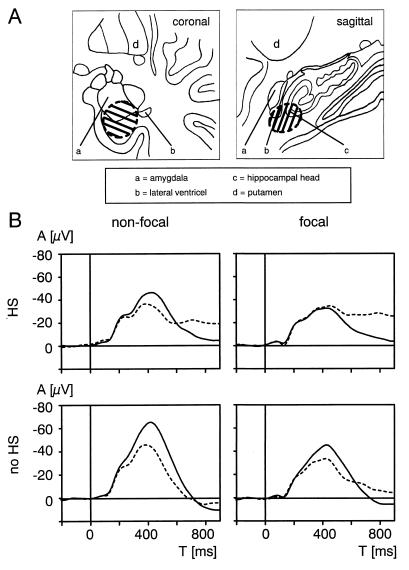
Figure 1.
Area of electrode locations at which maximal AMTL-N400s to words were recorded and grand averages of AMTL-N400s. (A) Schematics of recording sites of AMTL-N400s to words. Hatched area indicates the area in which maximal AMTL-N400 potentials were recorded in all patients. (B) Grand averages of AMTL-N400s in the nonfocal and focal temporal lobe in patients with extrahippocampal lesions and without hippocampal sclerosis (n = 21) as well as in patients with hippocampal sclerosis (n = 29). HS, hippocampal sclerosis. Solid line, first presentations; dashed line, word repetitions.
RESULTS
First presentations and repetitions of words elicited a negative component peaking around 400 ms (AMTL-N400) in the anterior medial temporal lobes (see Fig. 2). On both sides this potential was confined to the most anterior depth electrode contacts situated as a rule at the caudal edge of amygdala or hippocampal head or, due to some variability of implantation trajectories, outside the hippocampus in the vicinity of the parahippocampal gyrus near the collateral sulcus. It could not be recorded with more posterior contacts situated within the hippocampal body. Thus, the hatched area in Fig. 1A encompasses the locations of all contacts with maximal AMTL-N400 amplitudes that were used for statistical analyses and grand averages.
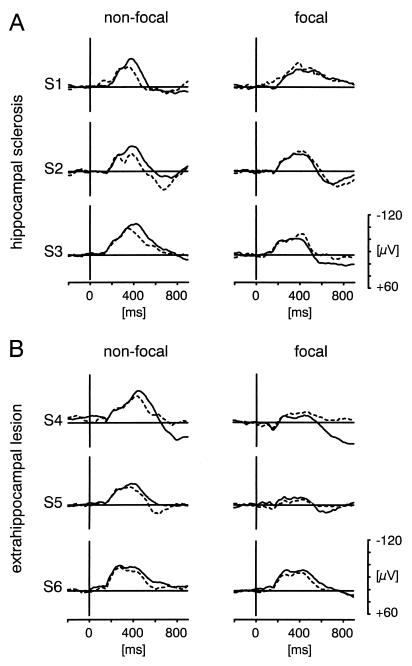
Figure 2.
Examples of AMTL-N400s to new and old words in six patients with temporal lobe epilepsy. On the nonfocal and focal side the AMTL-N400 from the contact with maximal amplitudes to first presentations was selected. Solid line, first presentations; dashed line, word repetitions. (A) Three patients with hippocampal sclerosis. (B) Three patients with extrahippocampal lesions and without hippocampal sclerosis. Note that in patient S4 there is no new-minus-old repetition effect on the focal side, although both AMTL-N400 to first presentations and repetitions are reduced.
In both patients with and those without hippocampal sclerosis there was no significant effect of different spans between first presentations and repetitions. In the hippocampal sclerosis group the interaction between lag and site of the epileptogenic focus just reached the 0.05 level of significance. Post-hoc t tests for paired samples, however, revealed no significant amplitude differences of AMTL-N400s to early and late repetitions on either side in both groups. Therefore, for the present study data was collapsed over both lags.
In patients without hippocampal sclerosis, AMTL-N400s were reduced in amplitude with repetition (F = 8.61; P < 0.01; Fig. 1B). Interactions between new-minus-old repetition effects and the side of measurement with respect to the epileptogenic focus were also significant (F = 9.80; P < 0.01) indicating reduced repetition effects on the focal side. MANOVA demonstrated that in these patients both AMTL-N400s to first presentations and to word repetitions were smaller in the epileptic temporal lobe (post-hoc univariate F tests for first presentations: df 1/19; F = 16.02; P < 0.005.; − for repetitions: df 1/19; F = 5.92; P < 0.05). By contrast, in patients with hippocampal sclerosis no significant new-minus-old repetition effect could be demonstrated by ANOVA for repeated measurements. There was, however, a significant repetition by side interaction (F = 28.04; P < 0.0005). Post-hoc comparison showed that repetition effects were missing only in the epileptic temporal lobe (mean new-minus-old amplitude differences on the focal vs. nonfocal side: −6.9: 9.9 μV; P < 0.0005). MANOVA demonstrated that in these patients with hippocampal sclerosis only AMTL-N400s to first presentations of words (df 1, 27; F = 12.72; P < 0.005) but not those elicited by repetitions (df 1, 27; F = 0.07; not significant) were reduced on the side of the epileptogenic focus. For the presence of hippocampal sclerosis as independent factor a significant effect was also revealed by MANOVA (P < 0.05). However, post-hoc univariate F tests showed that this was true only for AMTL-N400s to word repetitions on the focal side (df 1, 48; F = 4.92; P < 0.05) which were higher in amplitude in patients with hippocampal sclerosis (−55.4 ± 20.2 vs. −42.2 ± 21.4 μV; P < 0.05) but not for focal AMTL-N400s to first presentations. There was no difference between AMTL-N400s to new or old words in patients with and without hippocampal sclerosis on the contralateral side.
Patients with and those without hippocampal sclerosis displayed the same performance in the word recognition paradigm. The percentage of correct classifications of first presentations, of correct classifications of repetitions, and the calculated score of correct classifications of repetitions minus the incorrect or missing classifications of new words did not differ significantly. This result was true for both left and right TLE patients (see Table 2).
Table 2.
Performance in the word recognition paradigm
| Percentage of identified | HC sclerosis
|
No HC sclerosis
|
||
|---|---|---|---|---|
| TLE left | TLE right | TLE left | TLE right | |
| First presentations | 79 ± 16 | 87 ± 11 | 84 ± 9 | 88 ± 8 |
| Early repetitions | 74 ± 13 | 71 ± 17 | 69 ± 14 | 79 ± 18 |
| Late repetitions | 68 ± 15 | 67 ± 20 | 65 ± 17 | 70 ± 23 |
| All repetitions | 69 ± 17 | 69 ± 19 | 69 ± 14 | 73 ± 14 |
| Repetitions − errors | 39 ± 54 | 62 ± 44 | 53 ± 31 | 70 ± 32 |
The table contains means and standard deviations. HC, hippocampal.
Because of the lack of significant differences in performance between the four patients subgroups, it seemed justifiable to collapse all patients into a single group for the analysis of correlations between performance data and AMTL-N400 amplitudes. For all patients the number of correctly identified first representations was correlated only with left AMTL-N400s to first presentations but not to repetitions, whereas the number of correctly identified repetitions was correlated with left AMTL-N400s to first presentations and with the new-minus-old amplitude differences on the left but not right side (see Table 3).
Table 3.
Correlation between left and right AMTL-N400s and performance in the ERP paradigm
| No. of identified | Left temporal lobe AMTL-N400 to
|
Right temporal lobe AMTL-N400 to
|
||||||
|---|---|---|---|---|---|---|---|---|
| First presentations | Early R (Δ new-old) | Late R (Δ new-old) | All R (Δ new-old) | First presentations | Early R (Δ new-old) | Late R (Δ new-old) | All R (Δ new-old) | |
| First | r = 0.32* | r = 0.25 | r = 0.17 | r = 0.23 | r = −0.02 | r = −0.04 | r = −0.24 | r = 0.16 |
| presentations | (r = 0.13) | (r = 0.23) | (r = 0.18) | (r = −0.08) | (r = 0.16) | (r = −0.02) | ||
| Early R | r = 0.34* | r = 0.05 | r = 0.10 | r = 0.14 | r = 0.17 | r = −0.03 | r = −0.07 | r = −0.09 |
| (r = 0.43**) | (r = 0.43**) | (r = 0.35*) | (r = 0.15) | (r = 0.24) | (r = −0.07) | |||
| Late R | r = 0.28 | r = −0.03 | r = −0.01 | r = 0.04 | r = 0.09 | r = −0.11 | r = −0.07 | r = 0.09 |
| (r = 0.33*) | (r = 0.38*) | (r = 0.37*) | (r = 0.16) | (r = 0.23) | (r = −0.07) | |||
| All R | r = 0.32* | r = −0.03 | r = 0.04 | r = −0.01 | r = 0.10 | r = −0.05 | r = −0.07 | r = 0.23 |
| (r = 0.42**) | (r = 0.42**) | (r = 0.46**) | (r = 0.16) | (r = 0.23) | (r = 0.12) | |||
The table contains correlation coefficients for correlations between AMTL-N400 amplitudes as well as repetition effects (Δ new-old) and the number of identified first presentations, early, late, and all repetitions (R).
*P < 0.05.
**P < 0.01.
DISCUSSION
Maximal AMTL-N400s to words were recorded anterior to the hippocampus proper and near the amygdala. These locations were shown by McCarthy et al. (33) to be consistent with a generator within the parahippocampal region near the collateral sulcus. These authors found polarity inversions between the anterior fusiform and parahippocampal gyri and the white matter just superior to these sites but not near the amygdala (33). This finding may also explain why we did not find polarity inversions along the longitudinal axis of the hippocampus. The findings of Halgren et al. (31) are consistent with a generator in the rhinal cortex which covers the medial and basal surface of the amygdala and thus likewise suggest an extra- but parahippocampal generation of this component.
In the present study AMTL-N400s to words in the left and right temporal lobe were found to be reduced in amplitude on the focal side. The pattern of reduction, however, proved to be different in TLE patients with and without hippocampal sclerosis. In patients without hippocampal sclerosis AMTL-N400s to new and old items were reduced in the epileptic temporal lobe as was the new-minus-old repetition effect. Because the AMTL-N400 component has been shown to be generated in parahippocampal structures, it is not surprising that extrahippocampal lesions of the temporal lobe should interfere with its generation elicited by new as well as old stimuli. If the processing of either is more disturbed by epileptogenic foci in patients without hippocampal sclerosis cannot be differentiated by our data. However, because ERPs to both were affected, it may be speculated that extrahippocampal epileptogenic lesions can interfere with the detection of both novelty and repetition.
By contrast, in patients with hippocampal sclerosis only amplitudes of AMTL-N400s to new but not old words were reduced on the focal side. Here no new-minus-old repetition effects were found while they were present in the contralateral temporal lobe. This lack of repetition effects on the side of hippocampal sclerosis could theoretically be caused by changes in the generation of AMTL-N400s to new or old items, or both. If potentials elicited by old items were affected, this would represent another interpretational problem because word repetitions are not only known to elicit smaller AMTL-N400s than new stimuli but also large MTL-P600s. In a number of our patients, although not all, we also recorded this potential which had a more posterior distribution than the AMTL-N400. This finding is consistent with the findings of Smith et al. (29) whose data indicate different generators for both potentials. These authors also found that first presentations only elicit very small MTL-P600s, if any at all. For ERPs to word repetitions, however, it cannot be excluded that both components may overlap. Thus, if the epileptogenic process did alter ERPs to old items, one could not be sure which of both components was being affected. However, because AMTL-N400 to new words were significantly reduced in the epileptogenic temporal lobe but there was no difference between ipsi- and contralateral AMTL-N400s to old words, it must be concluded that ERPs to old words were not affected by hippocampal sclerosis. This result also explains why patients with and without hippocampal sclerosis only can be dissociated on the basis of the AMTL-N400 to repeated words: those to new words are attenuated on the focal side to the same extent in both patient groups, those to old words only in patients without hippocampal sclerosis. Thus, the only stimuli that proved to be sensitive for sclerosis of the hippocampus proper were set apart from others only by the fact that they were new, in the sense that they appeared for the first time in the given behavioral task. This, admittedly, is a restricted although important aspect of “novelty,” namely the first appearance of a known verbal stimulus in a new situational setting, and taps the formation of associational relations between stimuli or between stimuli and their “situational binding.” Because only ERPs to these new items were affected by hippocampal sclerosis, it can be concluded that the hippocampus proper contributes to the detection of the relative or situational novelty of verbal stimuli. This finding is consistent with the results of positron-emmision tomography studies: Tulving et al. (39) demonstrated activation of the hippocampal formation during encoding of novel stimuli; Dolan and Fletcher (40) found that although semantic changes in category–exemplar associations of word pairs induced left prefrontal activation, the left medial temporal cortex was activated when words were presented for the first time.
Our results do not suggest that parahippocampal structures do not contribute to novelty detection. In fact, although hippocampal sclerosis reduces AMTL-N400 amplitudes to new stimuli, extensive damage to or removal of the parahippocampal region would prevent its generation at all and would lead to even more dramatic neuropsychological deficits, consistent with the fact that the extensive medial temporal lobe damage of the amnesic patient H.M. lead to a more profound amnesia than the lesions restricted to the CA1 area of the hippocampus in patient R.B (41). However, our findings do show that the hippocampus proper is involved in the processing of stimulus novelty. Thus, while AMTL-N400s are generated parahippocampally, the hippocampal response to verbal novelty may contribute to the parahippocampal excitation generating AMTL-N400s and amplify potentials elicited by new verbal stimuli.
The finding that the patients’ performance was correlated with AMTL-N400s to first presentations and new-minus-old amplitude differences only in the left temporal lobe is consistent with earlier results published by our group (36). Yet patients with hippocampal sclerosis on the left side were not characterized by significantly worse performance. This result might indicate that either hippocampal formation could contribute equally to the discrimination between new and old words or that processes reflected in new-minus-old repetition effects may not be necessary for this discrimination (42). Nevertheless, the disturbance of novelty detection by hippocampal sclerosis may well have consequences for the patients’ memory performance. Preoperative memory deficits of TLE patients and changes in memory performance after temporal lobectomy have been shown to be associated with hippocampal atrophy and sclerosis (43, 44). In the present study, patients with hippocampal sclerosis also performed significantly worse in verbal and nonverbal memory tests than those without. Together with our previous finding that left AMTL-N400s to first presentations of words predict delayed verbal recall in the individual patient (36), these results suggest that the increment in temporo-mesial activity induced by hippocampal novelty detection may contribute to long-term storage of memory items: after all, why bother to learn something old? That the hippocampal processing of verbal novelty can be disturbed in both temporal lobes but left TLE patients exhibit more profound verbal memory deficits than patients with right TLE (45) may be explained by the interaction of the hippocampal system with neocortical areas specialized for language processes in the dominant hemisphere.
In conclusion, our findings show that hippocampal sclerosis has differential effects on medial temporal lobe ERPs elicited by new and old words in verbal recognition memory tasks. Extending the findings of previous studies that applied different techniques and found evidence of the participation of the medial temporal lobe system in novelty detection (21–26, 39, 40, 46), our data demonstrate that the hippocampus proper is involved in verbal novelty detection. Impairment of novelty detection thus may contribute to memory deficits in patients with hippocampal sclerosis.
Acknowledgments
We thank Drs. J. Schramm, E. Behrens, D. Van Roost, and J. Zentner, who performed the temporal lobe resections and selective amygdalo-hippocampectomies as well as Drs. O.D. Wiestler, I. Blümcke, and H.K. Wolf, who performed the histological analyses of the resected specimens. This study was supported by the Deutsche Forschungsgemeinschaft (projects El122/4-1 and Sonderforschungsbereich 400).
ABBREVIATIONS
- TLE
temporal lobe epilepsy
- AMTL
anterior medial temporal lobe
- ERP
event-related potential
References
- 1.Milner B. Clin Neurosurg. 1972;19:421–226. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- 2.Smith M, Milner B. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- 3.Hermann B P, Wyler A R, Steenman H, Richey E T. Cortex. 1988;24:245–253. doi: 10.1016/s0010-9452(88)80033-9. [DOI] [PubMed] [Google Scholar]
- 4.Zola-Morgan S, Squire L R. Science. 1990;250:288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]
- 5.Squire L R, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 6.Scoville W B, Milner B. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marr D. Philos Trans R Soc London B. 1971;262:23–82. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 8.Rawlins J N P. Behav Brain Sci. 1985;8:479–496. [Google Scholar]
- 9.Gray J A. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System. Oxford: Oxford Univ. Press; 1982. [Google Scholar]
- 10.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford Univ. Press; 1978. [Google Scholar]
- 11.Cohen N J, Squire L R. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 12.Squire L R. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 13.Jarrard L E. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- 14.Meunier M, Bachevalier J, Mishkin M, Murray E A. J Neuosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki W A, Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squire L R, Zola-Morgan S, Alvarez P. Behav Brain Sci. 1994;17:495–496. [Google Scholar]
- 17.Eichenbaum H, Otto T, Cohen N J. Behav Brain Sci. 1994;17:449–518. [Google Scholar]
- 18.Murray E A, Mishkin M. Science. 1985;228:604–606. doi: 10.1126/science.3983648. [DOI] [PubMed] [Google Scholar]
- 19.Murray E A, Gaffan D, Mishkin M. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunsey M, Eichenbaum H. Behav Neurosci. 1993;107:740–747. doi: 10.1037//0735-7044.107.5.740. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Miller E K, Desimone R. J Neurophysiol. 1991;69:1918–1929. doi: 10.1152/jn.1993.69.6.1918. [DOI] [PubMed] [Google Scholar]
- 22.Miller E K, Desimone R. Science. 1991;263:520–522. doi: 10.1126/science.8290960. [DOI] [PubMed] [Google Scholar]
- 23.Riches I P, Wilson F A, Brown M W. J Neurosci. 1991;11:1763–1779. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tulving E, Markowitsch H J, Craik F E, Habib R, Houle S. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 25.Stern C E, Corkin S, Gonzáles R G, Guimaraes A R, Baker J R, Jennings P J, Carr C A, Sugiura R M, Vedantham V, Rosen B R. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight R T. Nature (London) 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- 27.Smith M E, Halgren E. J Exp Psychol Learn Mem Cognit. 1989;15:50–60. doi: 10.1037//0278-7393.15.1.50. [DOI] [PubMed] [Google Scholar]
- 28.Rugg M D, Roberts R C, Potter D D, Pickles C D, Nagy M E. Brain. 1991;114:2313–2332. doi: 10.1093/brain/114.5.2313. [DOI] [PubMed] [Google Scholar]
- 29.Smith M E, Stapleton J M, Halgren E. Electroencephalogr Clin Neurophysiol. 1986;63:145–159. doi: 10.1016/0013-4694(86)90008-8. [DOI] [PubMed] [Google Scholar]
- 30.Heit G, Smith M E, Halgren E. Brain. 1990;113:1093–1112. doi: 10.1093/brain/113.4.1093. [DOI] [PubMed] [Google Scholar]
- 31.Halgren E, Baudena P, Heit G, Clarke M, Marinkovic K. J Physiol (Paris) 1994;88:1–50. doi: 10.1016/0928-4257(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 32.Halgren E, Baudena P, Heit G, Clarke M, Marinkovic K, Chauvel P. J Physiol (Paris) 1994;88:51–80. doi: 10.1016/0928-4257(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy G, Nobre A C, Bentin S, Spencer D D. J Neurosci. 1995;15:1080–1089. doi: 10.1523/JNEUROSCI.15-02-01080.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobre A C, McCarthy G. J Neurosci. 1995;15:1090–1098. doi: 10.1523/JNEUROSCI.15-02-01090.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grunwald T, Elger C E, Lehnertz K, Van Roost D, Heinze H J. Electroencephalogr Clin Neurophysiol. 1995;95:53–62. doi: 10.1016/0013-4694(95)00015-q. [DOI] [PubMed] [Google Scholar]
- 36.Elger C E, Grunwald T, Lehnertz K, Kutas M, Helmstaedter C, Brockhaus A, Van Roost D, Heinze H J. Neuropsychologia. 1997;35:657–668. doi: 10.1016/s0028-3932(96)00110-8. [DOI] [PubMed] [Google Scholar]
- 37.Helmstaedter C, Kurthen M, Linke D F, Elger C E. Brain. 1994;117:729–737. doi: 10.1093/brain/117.4.729. [DOI] [PubMed] [Google Scholar]
- 38.Duvernoy H M. The Human Hippocampus. Munich: Bergmann; 1988. [Google Scholar]
- 39.Tulving E, Markowitsch H J, Kapur S, Habib R, Houle S. NeuroReport. 1994;5:2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- 40.Dolan R J, Fletcher P C. Nature (London) 1997;388:582–585. doi: 10.1038/41561. [DOI] [PubMed] [Google Scholar]
- 41.Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rugg M D, Nagy M E. Electroencephalogr Clin Neurophysiol. 1989;72:395–406. doi: 10.1016/0013-4694(89)90045-x. [DOI] [PubMed] [Google Scholar]
- 43.Jones-Gotman, M., Brulot M., McMackin, D., Cendes, F., Andermann, F., Evans, A. & Peters, T. (1993) Epilepsia 34 Suppl. 6, 71 (abstr.).
- 44.Hermann B P, Seidenberg M, Dohan F C, Jr, Wyler A R, Haltiner A, Bobholz J, Perrine A. Neurosurgery. 1995;36:39–44. doi: 10.1227/00006123-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Hermann B P, Wyler A R, Steenman H, Richey E T. Cortex. 1988;24:245–253. doi: 10.1016/s0010-9452(88)80033-9. [DOI] [PubMed] [Google Scholar]
- 46.Fried I, MacDonald K A, Wilson C L. Neuron. 1997;18:753–765. doi: 10.1016/s0896-6273(00)80315-3. [DOI] [PubMed] [Google Scholar]