Abstract
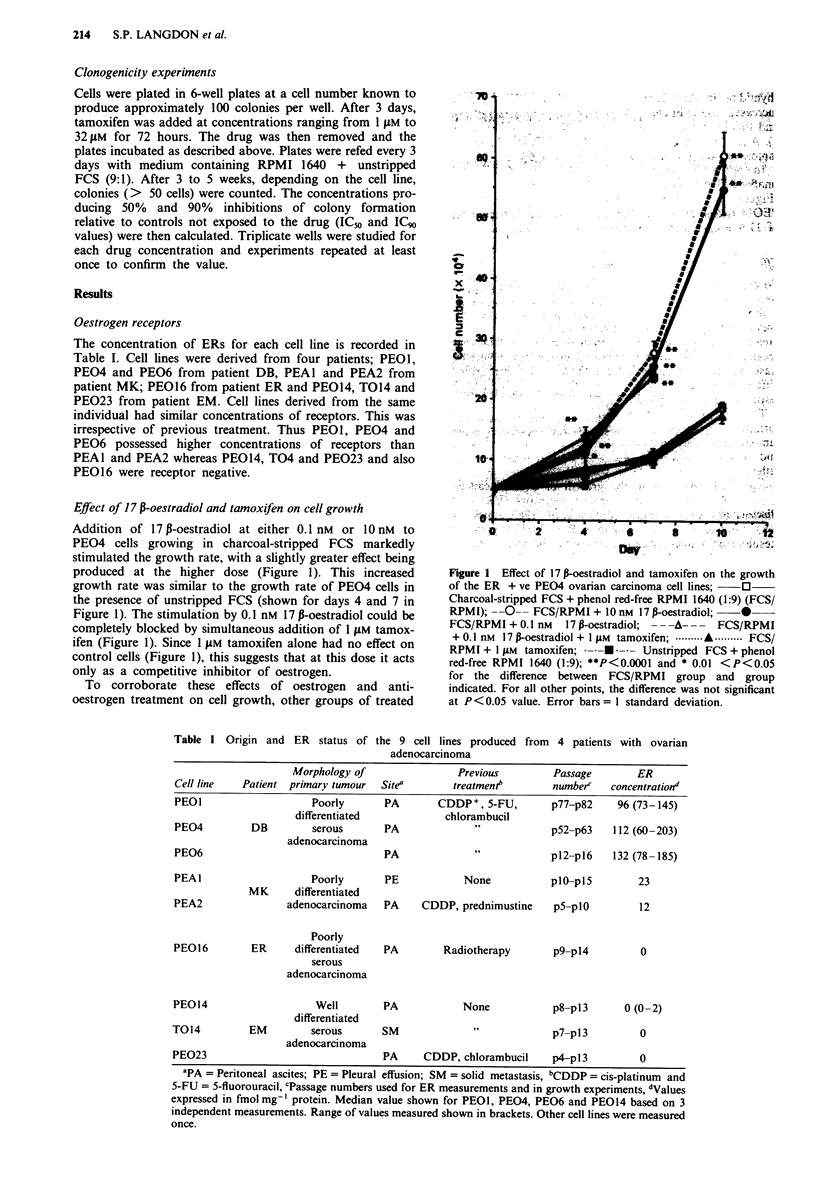
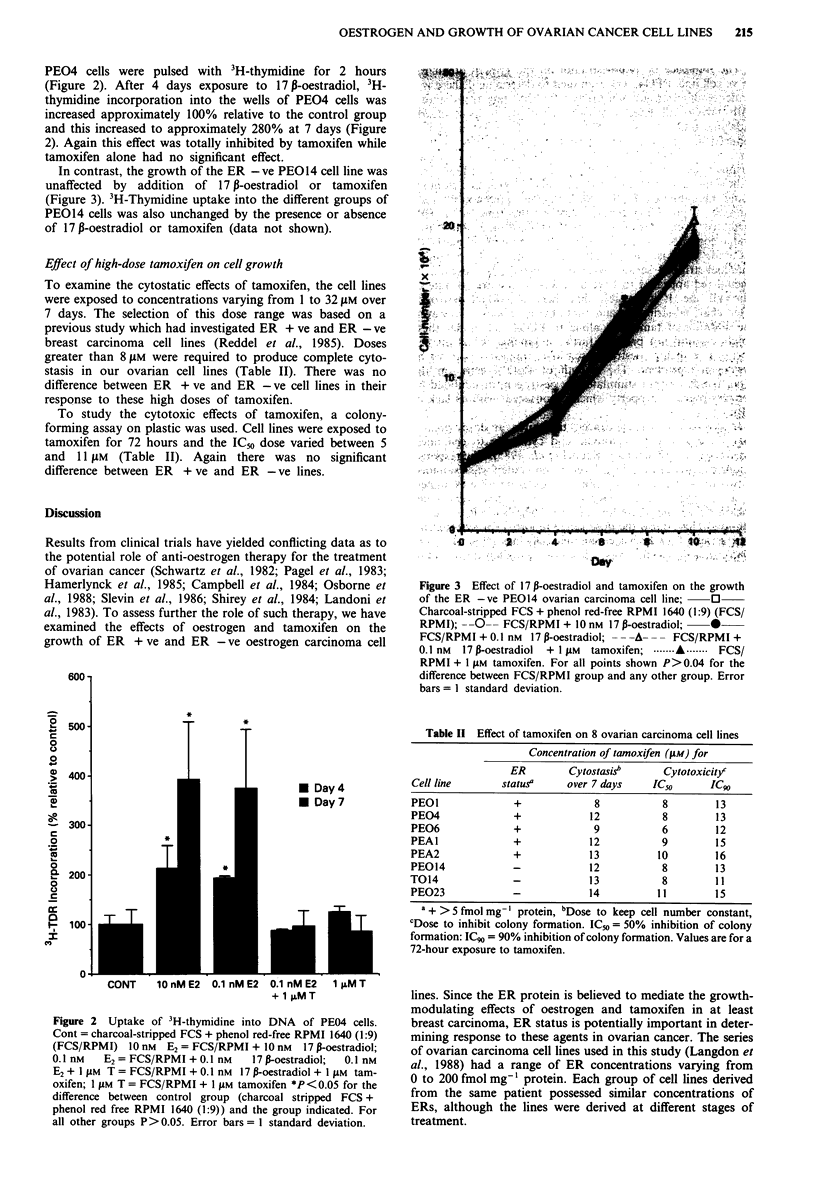
To assess the role of oestrogen regulation in the growth of ovarian cancer, we examined the effects of an oestrogen, 17 beta-oestradiol, and an anti-oestrogen, tamoxifen, on oestrogen receptor (ER) -positive and -negative human ovarian carcinoma cell lines. As measured by a dextran-coated charcoal adsorption assay, cell lines PEO1, PEO4 and PEO6 possessed moderate concentrations of ER (96-132 fmol mg-1 protein), PEA1 and PEA2 had low values (12-23 fmol mg-1 protein) and PEO14, TO14, PEO23 and PEO16 were ER-negative. Addition of 17 beta-oestradiol (10 nM or 0.1 nM) to the ER +ve cell line, PEO4, increased the growth rate. This oestrogen stimulation could be blocked by 1 microM tamoxifen. In contrast, the growth rate of the ER -ve cell line PEO14 was unaffected by the addition of 17 beta-oestradiol or tamoxifen. Concentrations of tamoxifen in excess of 8 microM were required to produce complete cytostasis in all lines. This concentration of tamoxifen over 72 hours also inhibited 50% colony formation when cells were plated on plastic. These data indicate that some ovarian carcinoma cell lines contain ER and their growth can be sensitive to oestrogen and anti-oestrogen modulation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fabian C., Sternson L., El-Serafi M., Cain L., Hearne E. Clinical pharmacology of tamoxifen in patients with breast cancer: correlation with clinical data. Cancer. 1981 Aug 15;48(4):876–882. doi: 10.1002/1097-0142(19810815)48:4<876::aid-cncr2820480403>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ford L. C., Berek J. S., Lagasse L. D., Hacker N. F., Heins Y., Esmailian F., Leuchter R. S., DeLange R. J. Estrogen and progesterone receptors in ovarian neoplasms. Gynecol Oncol. 1983 Jun;15(3):299–304. doi: 10.1016/0090-8258(83)90047-1. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Young R. C., McKoy W. M., Grotzinger K. R., Green J. A., Chu E. W., Whang-Peng J., Rogan A. M., Green W. R., Ozols R. F. Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983 Nov;43(11):5379–5389. [PubMed] [Google Scholar]
- Hawkins R. A., Black R., Steele R. J., Dixon J. M., Forrest A. P. Oestrogen receptor concentration in primary breast cancer and axillary node metastases. Breast Cancer Res Treat. 1981;1(3):245–251. doi: 10.1007/BF01806264. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Hill A., Freedman B. A simple method for the determination of oestrogen receptor concentrations in breast tumours and other tissues. Clin Chim Acta. 1975 Oct 15;64(2):203–210. doi: 10.1016/0009-8981(75)90202-8. [DOI] [PubMed] [Google Scholar]
- Jones L. A., Edwards C. L., Freedman R. S., Tan M. T., Gallager H. S. Estrogen and progesterone receptor titers in primary epithelial ovarian carcinomas. Int J Cancer. 1983 Nov 15;32(5):567–571. doi: 10.1002/ijc.2910320508. [DOI] [PubMed] [Google Scholar]
- Langdon S. P., Lawrie S. S., Hay F. G., Hawkes M. M., McDonald A., Hayward I. P., Schol D. J., Hilgers J., Leonard R. C., Smyth J. F. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988 Nov 1;48(21):6166–6172. [PubMed] [Google Scholar]
- Osborne R. J., Malik S. T., Slevin M. L., Harvey V. J., Spona J., Salzer H., Williams C. J. Tamoxifen in refractory ovarian cancer: the use of a loading dose schedule. Br J Cancer. 1988 Jan;57(1):115–116. doi: 10.1038/bjc.1988.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollow K., Schmidt-Matthiesen A., Hoffmann G., Schweikhart G., Kreienberg R., Manz B., Grill H. J. 3H-estradiol and 3H-R5020 binding in cytosols of normal and neoplastic human ovarian tissue. Int J Cancer. 1983 May 15;31(5):603–608. doi: 10.1002/ijc.2910310512. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Hall R. E., Sutherland R. L. Differential sensitivity of human breast cancer cell lines to the growth-inhibitory effects of tamoxifen. Cancer Res. 1985 Apr;45(4):1525–1531. [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Sutherland R. L. Factors affecting the sensitivity of T-47D human breast cancer cells to tamoxifen. Cancer Res. 1984 Jun;44(6):2398–2405. [PubMed] [Google Scholar]
- Runge H. M., Neumann H. A., Bauknecht T., Pfleiderer A. Growth patterns and hormonal sensitivity of primary tumor, abdominal metastasis and ascitic fluid from human epithelial ovarian carcinomas in the tumor colony-forming assay. Eur J Cancer Clin Oncol. 1986 Jun;22(6):691–696. doi: 10.1016/0277-5379(86)90167-7. [DOI] [PubMed] [Google Scholar]
- Runge H. M., Teufel G., Neulen J., Geyer H., Pfleiderer A. In vitro responsiveness of ovarian epithelial carcinomas to endocrine therapy. Cancer Chemother Pharmacol. 1986;16(1):58–63. doi: 10.1007/BF00255287. [DOI] [PubMed] [Google Scholar]
- Schwartz P. E., Keating G., MacLusky N., Naftolin F., Eisenfeld A. Tamoxifen therapy for advanced ovarian cancer. Obstet Gynecol. 1982 May;59(5):583–588. [PubMed] [Google Scholar]
- Slevin M. L., Harvey V. J., Osborne R. J., Shepherd J. H., Williams C. J., Mead G. M. A phase II study of tamoxifen in ovarian cancer. Eur J Cancer Clin Oncol. 1986 Mar;22(3):309–312. doi: 10.1016/0277-5379(86)90396-2. [DOI] [PubMed] [Google Scholar]
- Slotman B. J., Rao B. R. Ovarian cancer (review). Etiology, diagnosis, prognosis, surgery, radiotherapy, chemotherapy and endocrine therapy. Anticancer Res. 1988 May-Jun;8(3):417–434. [PubMed] [Google Scholar]
- Stanley E. R., Palmer R. E., Sohn U. Development of methods for the quantitative in vitro analysis of androgen-dependent and autonomous Shionogi carcinoma 115 cells. Cell. 1977 Jan;10(1):35–44. doi: 10.1016/0092-8674(77)90137-4. [DOI] [PubMed] [Google Scholar]
- Sutherland R. L., Hall R. E., Taylor I. W. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res. 1983 Sep;43(9):3998–4006. [PubMed] [Google Scholar]


