Abstract
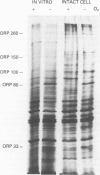
Cultured cells maintained in very low oxygen levels alter their structure, metabolism and genetic expression. Culture conditions for cells were modified to minimise variation of nutrients and to allow normal survival levels after 24 h of hypoxic exposure. Under these hypoxic conditions, glucose consumption and lactate production rates were similar to aerobic rates until about 12 h after which the hypoxic rates increased. DNA and protein synthesis rates are continuously inhibited to about 48% or 55% of the respective aerobic rates. During this period of decreased protein synthesis, a set of proteins termed oxygen regulated proteins (ORPs), exhibits enhanced relative synthesis. The molecular weights of the five major ORPs are approximately 260, 150, 100, 80 and 33 kDa. While increased relative synthesis of oxygen regulated proteins is partly due to increased levels of mRNA which encode these proteins, the mechanism of enhanced synthesis of ORPs may be more complex.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Born R., Hug O., Trott K. R. The effect of prolonged hypoxia on growth and viability of Chinese hamster cells. Int J Radiat Oncol Biol Phys. 1976 Jul-Aug;1(7-8):687–697. doi: 10.1016/0360-3016(76)90151-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carroll J. J., Smith N., Babson A. L. A colorimetric serum glucose determination using hexokinase and glucose-6-phosphate dehydrogenase. Biochem Med. 1970 Sep;4(2):171–180. doi: 10.1016/0006-2944(70)90093-1. [DOI] [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Dietz A. A., Lubrano T. Separation and quantitation of lactic dehydrogenase isoenzymes by disc electrophoresis. Anal Biochem. 1967 Aug;20(2):246–257. doi: 10.1016/0003-2697(67)90030-9. [DOI] [PubMed] [Google Scholar]
- Freudenberg H., Mager J. Studies on the mechanism of the inhibition of protein synthesis induced by intracellular ATP depletion. Biochim Biophys Acta. 1971 Mar 25;232(3):537–555. doi: 10.1016/0005-2787(71)90608-3. [DOI] [PubMed] [Google Scholar]
- Freyer J. P., Sutherland R. M. A reduction in the in situ rates of oxygen and glucose consumption of cells in EMT6/Ro spheroids during growth. J Cell Physiol. 1985 Sep;124(3):516–524. doi: 10.1002/jcp.1041240323. [DOI] [PubMed] [Google Scholar]
- Gerweck L. E., Nygaard T. G., Burlett M. Response of cells to hyperthermia under acute and chronic hypoxic conditions. Cancer Res. 1979 Mar;39(3):966–972. [PubMed] [Google Scholar]
- Gillies R. J., Ogino T., Shulman R. G., Ward D. C. 31P nuclear magnetic resonance evidence for the regulation of intracellular pH by Ehrlich ascites tumor cells. J Cell Biol. 1982 Oct;95(1):24–28. doi: 10.1083/jcb.95.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. Induction characteristics of oxygen regulated proteins. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1287–1290. doi: 10.1016/0360-3016(86)90155-0. [DOI] [PubMed] [Google Scholar]
- Hendershot L. M., Ting J., Lee A. S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol Cell Biol. 1988 Oct;8(10):4250–4256. doi: 10.1128/mcb.8.10.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Jacobson E. A., Hutchinson K., Inch W. R., Tustanoff E. R. Ultrastructural changes in V79 hamster lung fibroblasts during hypoxic exposure. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(1):23–43. doi: 10.1007/BF02912082. [DOI] [PubMed] [Google Scholar]
- Johnstone S. A., Schurch S., McIver D. J., Jacobson E. A., Tustanoff E. R. Membrane glycoprotein and surface free energy changes in hypoxic fibroblast cells. Biochim Biophys Acta. 1985 May 14;815(2):159–169. doi: 10.1016/0005-2736(85)90284-6. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Granger B. L. Preparation and assay of the intermediate filament proteins desmin and vimentin. Methods Enzymol. 1982;85(Pt B):488–508. doi: 10.1016/0076-6879(82)85048-9. [DOI] [PubMed] [Google Scholar]
- Li G. C., Shrieve D. C. Thermal tolerance and specific protein synthesis in Chinese hamster fibroblasts exposed to prolonged hypoxia. Exp Cell Res. 1982 Dec;142(2):464–468. doi: 10.1016/0014-4827(82)90390-1. [DOI] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Michael B. D., Adams G. E., Hewitt H. B., Jones W. B., Watts M. E. A posteffect of oxygen in irradiated bacteria: a submillisecond fast mixing study. Radiat Res. 1973 May;54(2):239–251. [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Lewis J. A., Huvos P., Henshaw E. C., Clemens M. J. The effects of amino acid starvation on regulation of polypeptide chain initiation in Ehrlich ascites tumor cells. J Biol Chem. 1980 Feb 25;255(4):1486–1491. [PubMed] [Google Scholar]
- Panniers R., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in heat-shocked Ehrlich ascites tumour cells. Eur J Biochem. 1984 Apr 2;140(1):209–214. doi: 10.1111/j.1432-1033.1984.tb08088.x. [DOI] [PubMed] [Google Scholar]
- Panniers R., Stewart E. B., Merrick W. C., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in heat-shocked Ehrlich cells involves reduction of eukaryotic initiation factor 4F activity. J Biol Chem. 1985 Aug 15;260(17):9648–9653. [PubMed] [Google Scholar]
- Peterson J. I., Young D. S. Evaluation of the hexokinase-glucose-6-phosphate dehydrogenase method of determination of glucose in urine. Anal Biochem. 1968 May;23(2):301–316. doi: 10.1016/0003-2697(68)90361-8. [DOI] [PubMed] [Google Scholar]
- Pettersen E. O., Juul N. O., Rønning O. W. Regulation of protein metabolism of human cells during and after acute hypoxia. Cancer Res. 1986 Sep;46(9):4346–4351. [PubMed] [Google Scholar]
- Pouysségur J., Yamada K. M. Isolation and immunological characterization of a glucose-regulated fibroblast cell surface glycoprotein and its nonglycosylated precursor. Cell. 1978 Jan;13(1):139–140. doi: 10.1016/0092-8674(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Probst H., Schiffer H., Gekeler V., Kienzle-Pfeilsticker H., Stropp U., Stötzer K. E., Frenzel-Stötzer I. Oxygen dependent regulation of DNA synthesis and growth of Ehrlich ascites tumor cells in vitro and in vivo. Cancer Res. 1988 Apr 15;48(8):2053–2060. [PubMed] [Google Scholar]
- Rice G. C., Hoy C., Schimke R. T. Transient hypoxia enhances the frequency of dihydrofolate reductase gene amplification in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5978–5982. doi: 10.1073/pnas.83.16.5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. C., Spiro I. J., Ling C. C. Detection of S-phase overreplication following chronic hypoxia using a monoclonal anti-BrdUrd. Int J Radiat Oncol Biol Phys. 1985 Oct;11(10):1817–1822. doi: 10.1016/0360-3016(85)90038-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. C., Rush B. F. An enzymatic-spectrophotometric determination of pyruvic and lactic acid in blood. Methodologic aspects. Clin Chem. 1966 May;12(5):299–307. [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrieve D. C., Deen D. F., Harris J. W. Effects of extreme hypoxia on the growth and viability of EMT6/SF mouse tumor cells in vitro. Cancer Res. 1983 Aug;43(8):3521–3527. [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T., Shen J., Johnson R. J. Association between the mammalian 110,000-dalton heat-shock protein and nucleoli. J Cell Biol. 1983 Nov;97(5 Pt 1):1389–1395. doi: 10.1083/jcb.97.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. M., Eddy H. A., Bareham B., Reich K., Vanantwerp D. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1225–1230. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Keng P., Conroy P. J., McDermott D., Bareham B. J., Passalacqua W. In vitro hypoxic cytotoxicity of nitroimidazoles: uptake and cell cycle phase specificity. Int J Radiat Oncol Biol Phys. 1982 Mar-Apr;8(3-4):745–748. doi: 10.1016/0360-3016(82)90726-x. [DOI] [PubMed] [Google Scholar]
- Teicher B. A., Lazo J. S., Sartorelli A. C. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981 Jan;41(1):73–81. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]
- Wilson R. E., Keng P. C., Sutherland R. M. Drug resistance in Chinese hamster ovary cells during recovery from severe hypoxia. J Natl Cancer Inst. 1989 Aug 16;81(16):1235–1240. doi: 10.1093/jnci/81.16.1235. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Sutherland R. M. Enhanced synthesis of specific proteins, RNA, and DNA caused by hypoxia and reoxygenation. Int J Radiat Oncol Biol Phys. 1989 Apr;16(4):957–961. doi: 10.1016/0360-3016(89)90895-x. [DOI] [PubMed] [Google Scholar]