Abstract
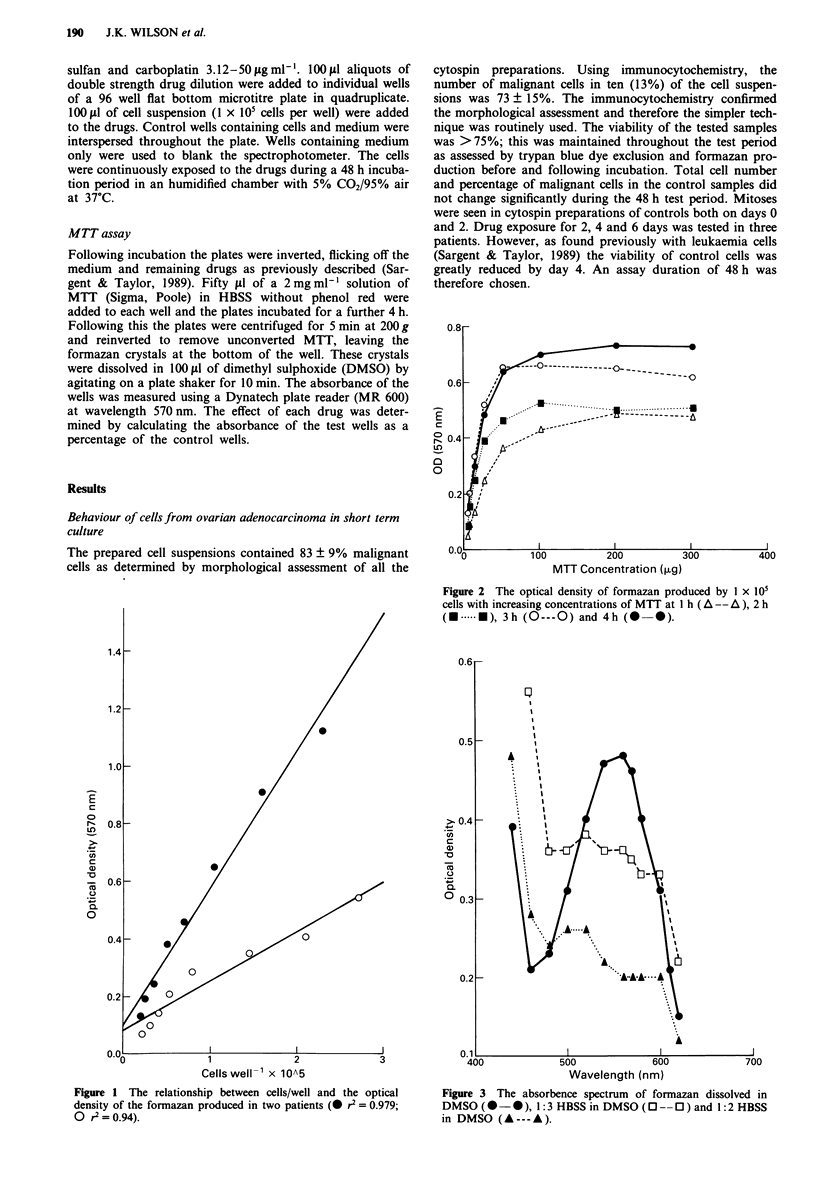
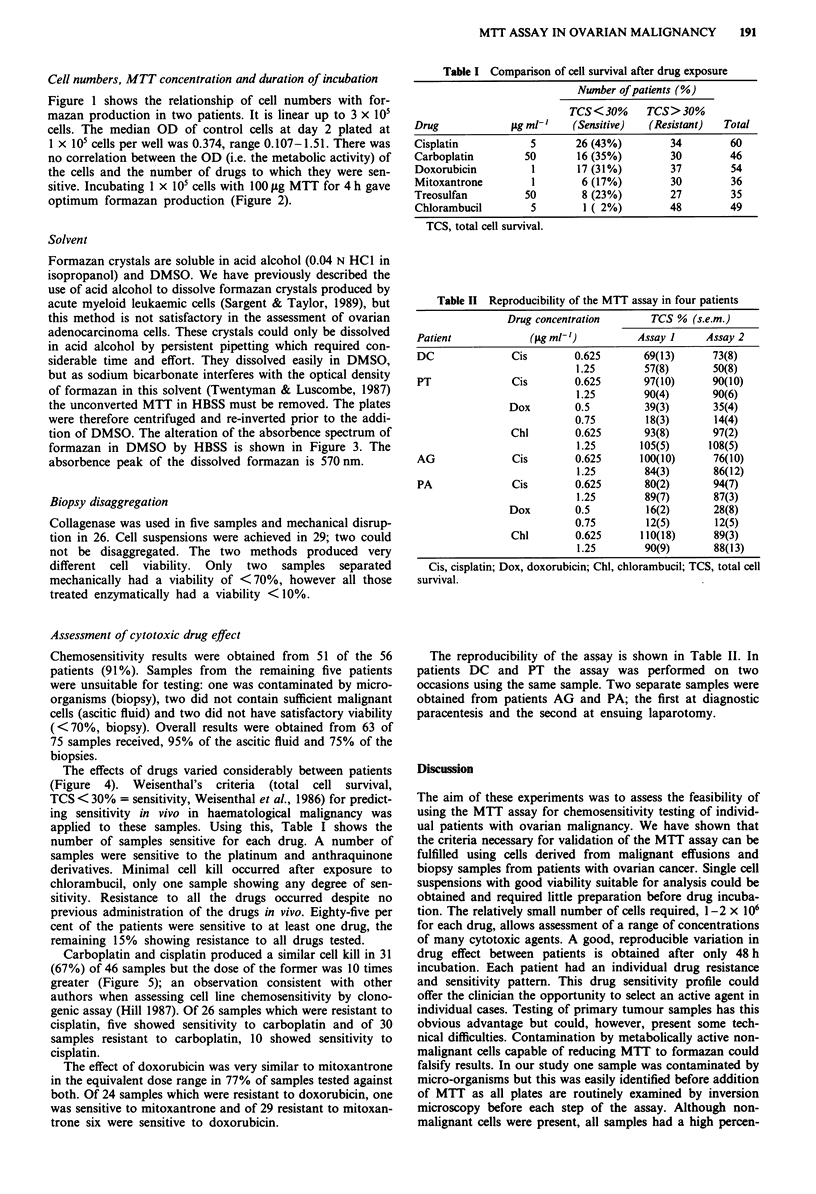
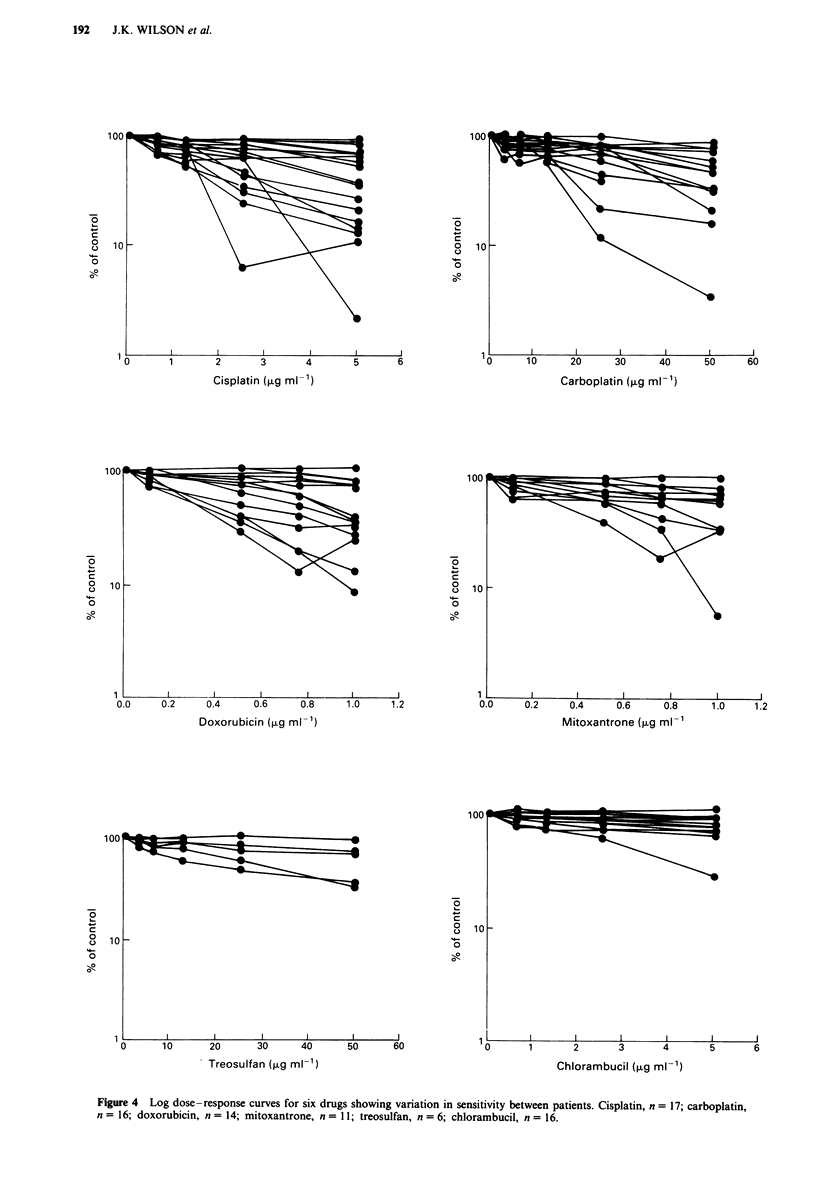
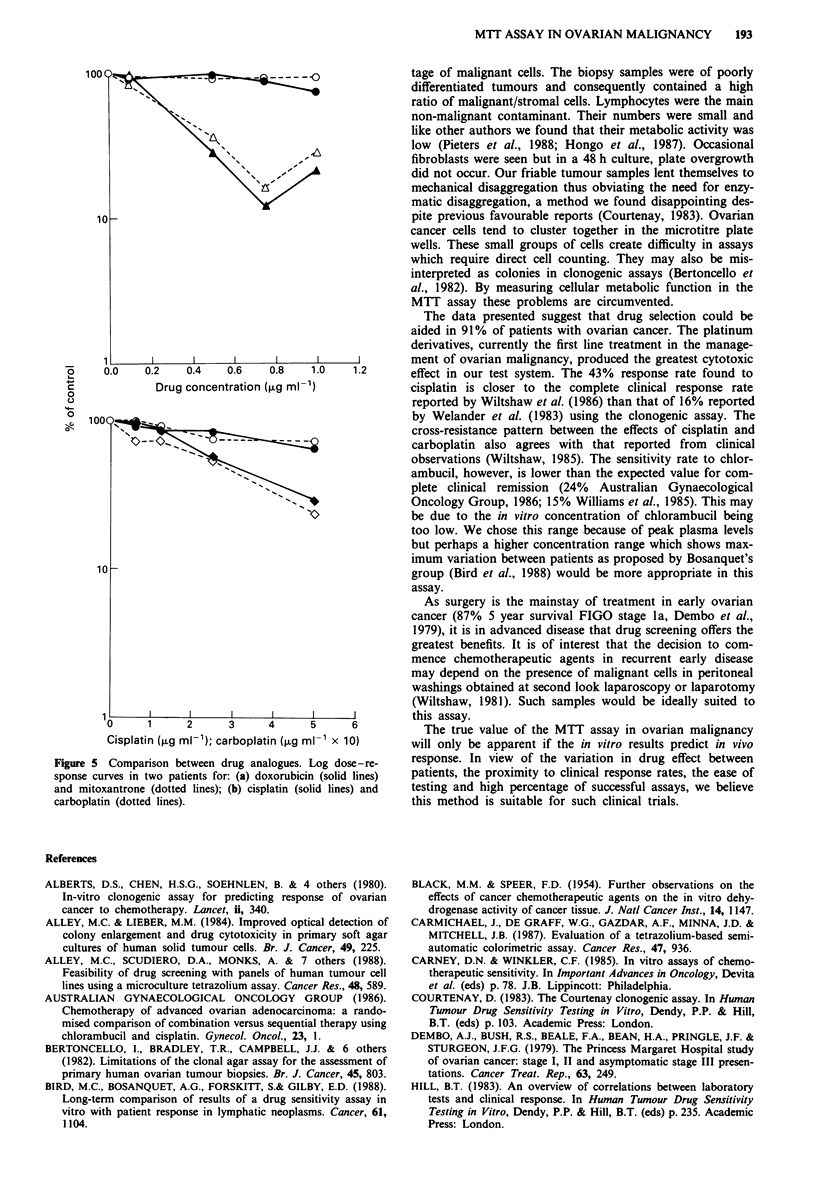
We assess the feasibility of using the MTT assay as a measure of cell viability in chemosensitivity testing in ovarian malignancy. The assay utilises the conversion of the tetrazolium salt MTT to formazan by dehydrogenase enzymes in living cells. We show that the optical density of the formazan produced from MTT is directly proportional to the number of live cells tested. Optimum MTT conversion occurred after 4 h incubation and dimethyl sulphoxide was found to be the most suitable solvent for the formazan. Seventy-five samples of ascitic fluid and/or solid tumour were collected from 56 patients with FIGO stage III-IV ovarian adenocarcinoma. Malignant cell suspensions with a viability greater than 75% were prepared from 95% of ascitic fluid and 75% of biopsy samples by simple techniques. The effect of cytotoxic drugs was assessed in 91% of patients included in the study. Variation in drug effect between patients was evident following a 48 h incubation period and was reproducible. Overall platinum and anthraquinone analogues produced the greater effect but resistance did occur. Our results mirrored reported clinical response rates. Only one sample tested against chlorambucil showed any drug effect. As this assay produces results in a high percentage of tests and is rapid and simple it appears suitable for prospective clinical trials to correlate the in vitro results with in vivo response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts D. S., Samon S. E., Chen H. S., Surwit E. A., Soehnlen B., Young L., Moon T. E. In-vitro clonogenic assay for predicting response of ovarian cancer to chemotherapy. Lancet. 1980 Aug 16;2(8190):340–342. doi: 10.1016/s0140-6736(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Alley M. C., Lieber M. M. Improved optical detection of colony enlargement and drug cytotoxicity in primary soft agar cultures of human solid tumour cells. Br J Cancer. 1984 Feb;49(2):225–233. doi: 10.1038/bjc.1984.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley M. C., Scudiero D. A., Monks A., Hursey M. L., Czerwinski M. J., Fine D. L., Abbott B. J., Mayo J. G., Shoemaker R. H., Boyd M. R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988 Feb 1;48(3):589–601. [PubMed] [Google Scholar]
- BLACK M. M., SPEER F. D. Further observations on the effects of cancer chemotherapeutic agents on the in vitro dehydrogenase activity of cancer tissue. J Natl Cancer Inst. 1954 Apr;14(5):1147–1158. [PubMed] [Google Scholar]
- Bertoncello I., Bradley T. R., Campbell J. J., Day A. J., McDonald I. A., McLeish G. R., Quinn M. A., Rome R., Hodgson G. S. Limitations of the clonal agar assay for the assessment of primary human ovarian tumour biopsies. Br J Cancer. 1982 Jun;45(6):803–811. doi: 10.1038/bjc.1982.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. C., Bosanquet A. G., Forskitt S., Gilby E. D. Long-term comparison of results of a drug sensitivity assay in vitro with patient response in lymphatic neoplasms. Cancer. 1988 Mar 15;61(6):1104–1109. doi: 10.1002/1097-0142(19880315)61:6<1104::aid-cncr2820610609>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Dembo A. J., Bush R. S., Beale F. A., Bean H. A., Pringle J. F., Sturgeon J. F. The Princess Margaret Hospital study of ovarian cancer: stages I, II, and asymptomatic III presentations. Cancer Treat Rep. 1979 Feb;63(2):249–254. [PubMed] [Google Scholar]
- Hill B. T. In vitro screening of new drugs and analogues--specificity and selectivity. Cancer Treat Rev. 1987 Dec;14(3-4):197–202. doi: 10.1016/0305-7372(87)90008-9. [DOI] [PubMed] [Google Scholar]
- Hongo T., Fujii Y., Mizuno Y., Haraguchi S., Yoshida T. O. [Anticancer drug sensitivity test using the short-term microplate culture and MTT dye reduction assay]. Gan To Kagaku Ryoho. 1987 Feb;14(2):472–478. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pieters R., Huismans D. R., Leyva A., Veerman A. J. Adaptation of the rapid automated tetrazolium dye based (MTT) assay for chemosensitivity testing in childhood leukemia. Cancer Lett. 1988 Aug 30;41(3):323–332. doi: 10.1016/0304-3835(88)90294-7. [DOI] [PubMed] [Google Scholar]
- Sargent J. M., Taylor C. G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer. 1989 Aug;60(2):206–210. doi: 10.1038/bjc.1989.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Rees J. K. Chemosensitivity testing of fresh leukaemia cells using the MTT colorimetric assay. Br J Haematol. 1989 Jan;71(1):19–24. doi: 10.1111/j.1365-2141.1989.tb06268.x. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987 Sep;56(3):279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenthal L. M., Dill P. L., Finklestein J. Z., Duarte T. E., Baker J. A., Moran E. M. Laboratory detection of primary and acquired drug resistance in human lymphatic neoplasms. Cancer Treat Rep. 1986 Nov;70(11):1283–1295. [PubMed] [Google Scholar]
- Weisenthal L. M., Lippman M. E. Clonogenic and nonclonogenic in vitro chemosensitivity assays. Cancer Treat Rep. 1985 Jun;69(6):615–632. [PubMed] [Google Scholar]
- Welander C. E., Homesley H. D., Jobson V. W. In vitro chemotherapy testing of gynecologic tumors: basis for planning therapy? Am J Obstet Gynecol. 1983 Sep 15;147(2):188–195. doi: 10.1016/0002-9378(83)90114-x. [DOI] [PubMed] [Google Scholar]
- Williams C. J., Mead G. M., Macbeth F. R., Thompson J., Whitehouse J. M., MacDonald H., Harvey V. J., Slevin M. L., Lister T. A., Shepherd J. H. Cisplatin combination chemotherapy versus chlorambucil in advanced ovarian carcinoma: mature results of a randomized trial. J Clin Oncol. 1985 Nov;3(11):1455–1462. doi: 10.1200/JCO.1985.3.11.1455. [DOI] [PubMed] [Google Scholar]
- Wiltshaw E., Evans B., Rustin G., Gilbey E., Baker J., Barker G. A prospective randomized trial comparing high-dose cisplatin with low-dose cisplatin and chlorambucil in advanced ovarian carcinoma. J Clin Oncol. 1986 May;4(5):722–729. doi: 10.1200/JCO.1986.4.5.722. [DOI] [PubMed] [Google Scholar]
- Wiltshaw E. Ovarian trials at the Royal Marsden. Cancer Treat Rev. 1985 Sep;12 (Suppl A):67–71. doi: 10.1016/0305-7372(85)90020-9. [DOI] [PubMed] [Google Scholar]


