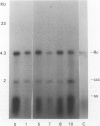
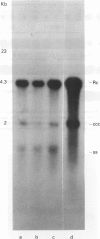
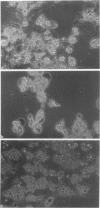
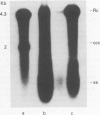
Abstract
The preparation of primary cultures of control and DHBV-infected duck hepatocytes from embryos and young ducklings is described. Cultures of both embryo and duckling hepatocytes secreted duck serum proteins. Cultures of hepatocytes established from ducklings maintained initial morphology for up to 3 weeks in culture and also exhibited high levels of metabolism of aflatoxin B1. Embryonic cell cultures rapidly lost ability to metabolise AFB1 and became overgrown by spindle-shaped cells. Both embryo and duckling cell cultures secreted infective DHBV, and had intracellular replicative forms of the virus. No integration of the virus into the duck genome was observed, and attempts to induce viral integration in the duckling hepatocytes using irradiation and aflatoxin B1 toxicity were unsuccessful. The results of the study lend further support to the suggestion that the rarity of liver cancer in DHBV-infected experimental ducks is related to an innate resistance of the hepatocytes to develop DHBV-DNA integration. Another possibility may be related to the lower oncogenic potential of the DHBV strain used for the study. However DHBV infected duckling hepatocytes would appear to offer a suitable material for studying viral replication and mechanisms of aflatoxin B1 toxicity during prolonged cell culture.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoola E. A. Synergism between hepatitis B virus and aflatoxin in hepatocellular carcinoma. IARC Sci Publ. 1984;(63):167–179. [PubMed] [Google Scholar]
- Brechot C., Pourcel C., Louise A., Rain B., Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980 Jul 31;286(5772):533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- Cova L., Hantz O., Arliaud-Gassin M., Chevalier A., Berthillon P., Boulay J., Jacquet C., Chomel B., Vitvitski L., Trepo C. Comparative study of DHBV DNA levels and endogenous DNA polymerase activity in naturally infected ducklings in France. J Virol Methods. 1985 Mar;10(3):251–260. doi: 10.1016/0166-0934(85)90065-5. [DOI] [PubMed] [Google Scholar]
- Cova L., Wild C. P., Mehrotra R., Turusov V., Shirai T., Lambert V., Jacquet C., Tomatis L., Trépo C., Montesano R. Contribution of aflatoxin B1 and hepatitis B virus infection in the induction of liver tumors in ducks. Cancer Res. 1990 Apr 1;50(7):2156–2163. [PubMed] [Google Scholar]
- Cullen J. M., Marion P. L., Sherman G. J., Hong X., Newbold J. E. Hepatic neoplasms in aflatoxin B1-treated, congenital duck hepatitis B virus-infected, and virus-free pekin ducks. Cancer Res. 1990 Jul 1;50(13):4072–4080. [PubMed] [Google Scholar]
- Fourel I., Gripon P., Hantz O., Cova L., Lambert V., Jacquet C., Watanabe K., Fox J., Guillouzo C., Trepo C. Prolonged duck hepatitis B virus replication in duck hepatocytes cocultivated with rat epithelial cells: a useful system for antiviral testing. Hepatology. 1989 Aug;10(2):186–191. doi: 10.1002/hep.1840100211. [DOI] [PubMed] [Google Scholar]
- Imazeki F., Yaginuma K., Omata M., Okuda K., Kobayashi M., Koike K. Integrated structures of duck hepatitis B virus DNA in hepatocellular carcinoma. J Virol. 1988 Mar;62(3):861–865. doi: 10.1128/jvi.62.3.861-865.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurohara S. S., Swensson N. L., Usselman J. A., George F. W., 3rd Response and recovery of liver to radiation as demonstrated by photoscans. Radiology. 1967 Jul;89(1):129–135. doi: 10.1148/89.1.129. [DOI] [PubMed] [Google Scholar]
- Marion P. L., Oshiro L. S., Regnery D. C., Scullard G. H., Robinson W. S. A virus in Beechey ground squirrels that is related to hepatitis B virus of humans. Proc Natl Acad Sci U S A. 1980 May;77(5):2941–2945. doi: 10.1073/pnas.77.5.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion P. L., Van Davelaar M. J., Knight S. S., Salazar F. H., Garcia G., Popper H., Robinson W. S. Hepatocellular carcinoma in ground squirrels persistently infected with ground squirrel hepatitis virus. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4543–4546. doi: 10.1073/pnas.83.12.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason W. S., Halpern M. S., England J. M., Seal G., Egan J., Coates L., Aldrich C., Summers J. Experimental transmission of duck hepatitis B virus. Virology. 1983 Dec;131(2):375–384. doi: 10.1016/0042-6822(83)90505-6. [DOI] [PubMed] [Google Scholar]
- Mason W. S., Seal G., Summers J. Virus of Pekin ducks with structural and biological relatedness to human hepatitis B virus. J Virol. 1980 Dec;36(3):829–836. doi: 10.1128/jvi.36.3.829-836.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss E. J., Judah D. J., Przybylski M., Neal G. E. Some mass-spectral and n.m.r. analytical studies of a glutathione conjugate of aflatoxin B1. Biochem J. 1983 Jan 15;210(1):227–233. doi: 10.1042/bj2100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal G. E., Judah D. J., Stirpe F., Patterson D. S. The formation of 2,3-dihydroxy-2,3-dihydro-aflatoxin B1 by the metabolism of aflatoxin B1 by liver microsomes isolated from certain avian and mammalian species and the possible role of this metabolite in the acute toxicity of aflatoxin B1. Toxicol Appl Pharmacol. 1981 May;58(3):431–437. doi: 10.1016/0041-008x(81)90095-8. [DOI] [PubMed] [Google Scholar]
- O'Connell A. P., Urban M. K., London W. T. Naturally occurring infection of Pekin duck embryos by duck hepatitis B virus. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1703–1706. doi: 10.1073/pnas.80.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olubuyide I. O., Atoba M. A., Ayoola E. A. Primary hepatocellular carcinoma in Africans. Trop Gastroenterol. 1986 Apr-May;7(2):43–48. [PubMed] [Google Scholar]
- Omata M., Uchiumi K., Ito Y., Yokosuka O., Mori J., Terao K., Wei-Fa Y., O'Connell A. P., London W. T., Okuda K. Duck hepatitis B virus and liver diseases. Gastroenterology. 1983 Aug;85(2):260–267. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scotto J., Hadchouel M., Hery C., Yvart J., Tiollais P., Brechot C. Detection of hepatitis B virus DNA in serum by a simple spot hybridization technique: comparison with results for other viral markers. Hepatology. 1983 May-Jun;3(3):279–284. doi: 10.1002/hep.1840030301. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. I. Effect of Ca 2+ on enzymatic dispersion of isolated, perfused liver. Exp Cell Res. 1972 Oct;74(2):450–454. doi: 10.1016/0014-4827(72)90400-4. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Shouval D., Sherman H. I., Hadziyannis S. J., Kew M. C. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981 Oct 29;305(18):1067–1073. doi: 10.1056/NEJM198110293051807. [DOI] [PubMed] [Google Scholar]
- Sinclair P. R., Granick S. Uroporphyrin formation induced by chlorinated hydrocarbons (lindane, polychlorinated biphenyls, tetrachlorodibenzo-p-dioxin). Requirements for endogenous iron, protein synthesis and drug-metabolizing activity. Biochem Biophys Res Commun. 1974 Nov 6;61(1):124–133. doi: 10.1016/0006-291x(74)90543-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Summers J., O'Connell A., Millman I. Genome of hepatitis B virus: restriction enzyme cleavage and structure of DNA extracted from Dane particles. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4597–4601. doi: 10.1073/pnas.72.11.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Smolec J. M., Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thézé N., Gripon P., Fourel I., Hantz O., Trepo C., Guguen-Guillouzo C. Maintenance of woodchuck hepatitis virus activity in woodchuck hepatocyte primary culture. J Gen Virol. 1987 Apr;68(Pt 4):1029–1039. doi: 10.1099/0022-1317-68-4-1029. [DOI] [PubMed] [Google Scholar]
- Tuttleman J. S., Pugh J. C., Summers J. W. In vitro experimental infection of primary duck hepatocyte cultures with duck hepatitis B virus. J Virol. 1986 Apr;58(1):17–25. doi: 10.1128/jvi.58.1.17-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Suzuki K., Okuda Y., Shikata T. In vitro transmission of duck hepatitis B virus to primary duck hepatocyte cultures. Hepatology. 1988 Jul-Aug;8(4):760–765. doi: 10.1002/hep.1840080410. [DOI] [PubMed] [Google Scholar]
- Urban M. K., O'Connell A. P., London W. T. Sequence of events in natural infection of Pekin duck embryos with duck hepatitis B virus. J Virol. 1985 Jul;55(1):16–22. doi: 10.1128/jvi.55.1.16-22.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka O., Omata M., Zhou Y. Z., Imazeki F., Okuda K. Duck hepatitis B virus DNA in liver and serum of Chinese ducks: integration of viral DNA in a hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5180–5184. doi: 10.1073/pnas.82.15.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]