Abstract
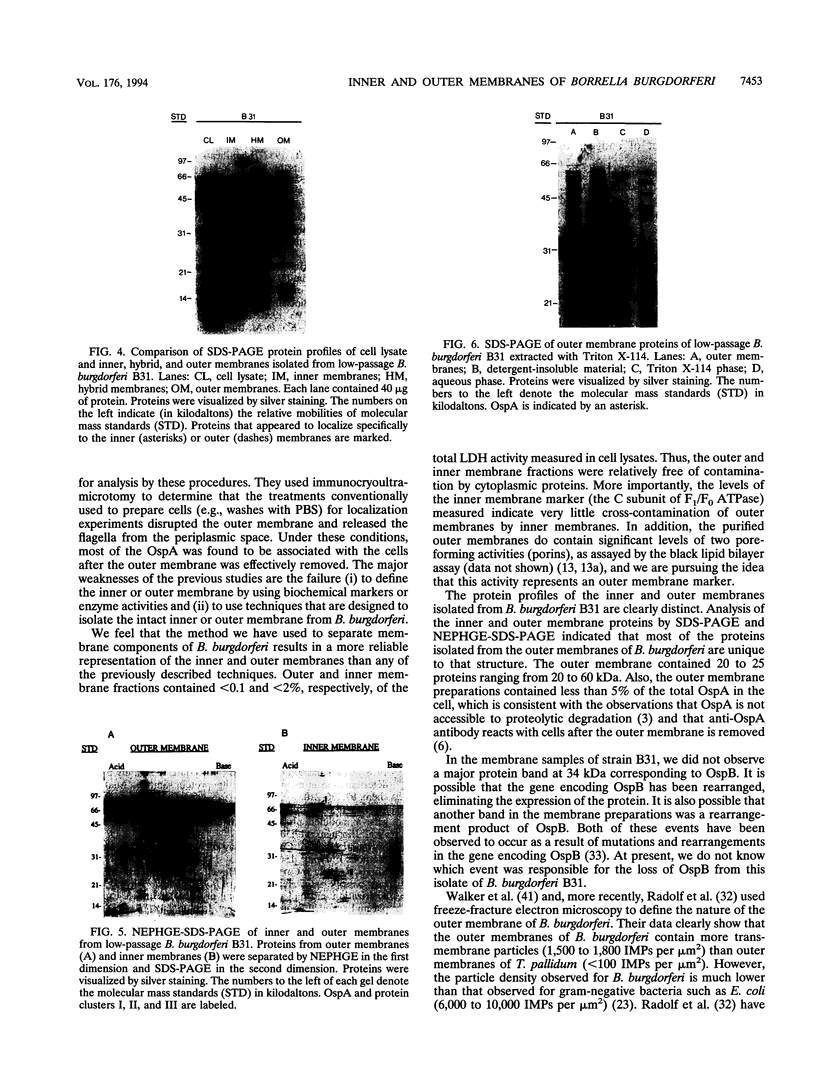
In order to characterize the protein composition of the outer membrane of Borrelia burgdorferi, we have isolated inner and outer membranes by using discontinuous sucrose density step gradients. Outer and inner membrane fractions isolated by this method contained less than 1 and 2%, respectively, of the total lactate dehydrogenase activity (soluble marker) in cell lysate. More importantly, the purified outer membranes contained less than 4% contamination by the C subunit of F1/F0 ATPase (inner membrane marker). Very little flagellin protein was present in the outer membrane sample. This indicated that the outer membranes were relatively free of contamination by cytoplasmic, inner membrane or flagellar components. The outer membrane fractions (rho = 1.19 g/cm3) contained 0.15 mg (dry weight) of protein per mg. Inner membrane samples (rho = 1.12 g/cm3) contained 0.60 mg (dry weight) of protein per mg. Freeze-fracture electron microscopy revealed that the outer membrane vesicles contained about 1,700 intramembranous particles per micron 2 while inner membrane densities for inner and outer membranes. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and nonequilibrium pH gel electrophoresis-SDS-PAGE analyses of inner and outer membrane samples revealed several proteins unique to the inner membrane and 20 proteins that localized specifically to the outer membrane. This analysis clearly shows that the inner and outer membranes isolated by this technique are unique structures.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Todd W. J. Lyme disease spirochetes and ixodid tick spirochetes share a common surface antigenic determinant defined by a monoclonal antibody. Infect Immun. 1983 Aug;41(2):795–804. doi: 10.1128/iai.41.2.795-804.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Coleman J. L. Chemical and biologic characterization of a lipopolysaccharide extracted from the Lyme disease spirochete (Borrelia burgdorferi). J Infect Dis. 1985 Jul;152(1):108–117. doi: 10.1093/infdis/152.1.108. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brusca J. S., McDowall A. W., Norgard M. V., Radolf J. D. Localization of outer surface proteins A and B in both the outer membrane and intracellular compartments of Borrelia burgdorferi. J Bacteriol. 1991 Dec;173(24):8004–8008. doi: 10.1128/jb.173.24.8004-8008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L., Beck G., Habicht G. S. Isolation of the outer envelope from Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):123–126. doi: 10.1016/s0176-6724(86)80112-2. [DOI] [PubMed] [Google Scholar]
- Ferdows M. S., Barbour A. G. Megabase-sized linear DNA in the bacterium Borrelia burgdorferi, the Lyme disease agent. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5969–5973. doi: 10.1073/pnas.86.15.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girvin M. E., Hermolin J., Pottorf R., Fillingame R. H. Organization of the F0 sector of Escherichia coli H+-ATPase: the polar loop region of subunit c extends from the cytoplasmic face of the membrane. Biochemistry. 1989 May 16;28(10):4340–4343. doi: 10.1021/bi00436a032. [DOI] [PubMed] [Google Scholar]
- Hofstad T., Kristoffersen T. Chemical characteristics of endotoxin from Bacteroides fragilis NCTC 9343. J Gen Microbiol. 1970 Apr;61(1):15–19. doi: 10.1099/00221287-61-1-15. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Hyde F. W., Rumpel C. M. Taxonomy of the Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):529–537. [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Marek N., Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984 Dec;20(6):1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [PubMed] [Google Scholar]
- Kasper D. L. Chemical and biological characterization of the lipopolysaccharide of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1976 Jul;134(1):59–66. doi: 10.1093/infdis/134.1.59. [DOI] [PubMed] [Google Scholar]
- Kotarski S. F., Salyers A. A. Isolation and characterization of outer membranes of Bacteroides thetaiotaomicron grown on different carbohydrates. J Bacteriol. 1984 Apr;158(1):102–109. doi: 10.1128/jb.158.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lovrich S. D., Callister S. M., Schmitz J. L., Alder J. D., Schell R. F. Borreliacidal activity of sera from hamsters infected with the Lyme disease spirochete. Infect Immun. 1991 Aug;59(8):2522–2528. doi: 10.1128/iai.59.8.2522-2528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft B. J., Jiang W., Munoz P., Dattwyler R. J., Gorevic P. D. Biochemical and immunological characterization of the surface proteins of Borrelia burgdorferi. Infect Immun. 1989 Nov;57(11):3637–3645. doi: 10.1128/iai.57.11.3637-3645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983 Mar 21;737(1):51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- Macy J. M., Ljungdahl L. G., Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol. 1978 Apr;134(1):84–91. doi: 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Norris S. J., Carter C. J., Howell J. K., Barbour A. G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992 Nov;60(11):4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørby J. G. Coupled assay of Na+,K+-ATPase activity. Methods Enzymol. 1988;156:116–119. doi: 10.1016/0076-6879(88)56014-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Radolf J. D., Bourell K. W., Akins D. R., Brusca J. S., Norgard M. V. Analysis of Borrelia burgdorferi membrane architecture by freeze-fracture electron microscopy. J Bacteriol. 1994 Jan;176(1):21–31. doi: 10.1128/jb.176.1.21-31.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Sambri V., Cevenini R. Comparative ability of various detergents to extract proteins of Borrelia burgdorferi and Borrelia hermsii. Microbiologica. 1991 Oct;14(4):307–313. [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S. Purification and characterization of NADH oxidase from Serpulina (Treponema) hyodysenteriae. J Bacteriol. 1993 May;175(10):2980–2987. doi: 10.1128/jb.175.10.2980-2987.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K., Rothenberg R. J., Barbour A. G. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987 Sep;55(9):2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unden G., Kröger A. Reconstitution of a functional electron-transfer chain from purified formate dehydrogenase and fumarate reductase complexes. Methods Enzymol. 1986;126:387–399. doi: 10.1016/s0076-6879(86)26039-5. [DOI] [PubMed] [Google Scholar]
- Walker E. M., Borenstein L. A., Blanco D. R., Miller J. N., Lovett M. A. Analysis of outer membrane ultrastructure of pathogenic Treponema and Borrelia species by freeze-fracture electron microscopy. J Bacteriol. 1991 Sep;173(17):5585–5588. doi: 10.1128/jb.173.17.5585-5588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]








