Abstract
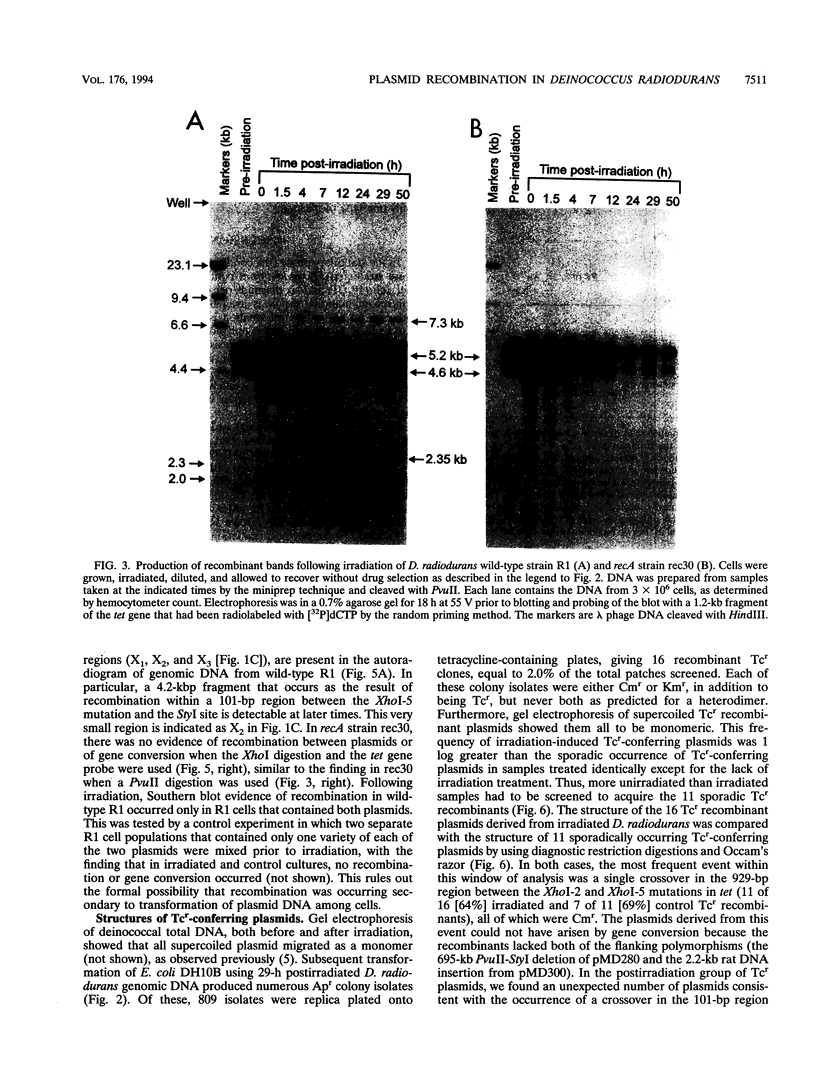
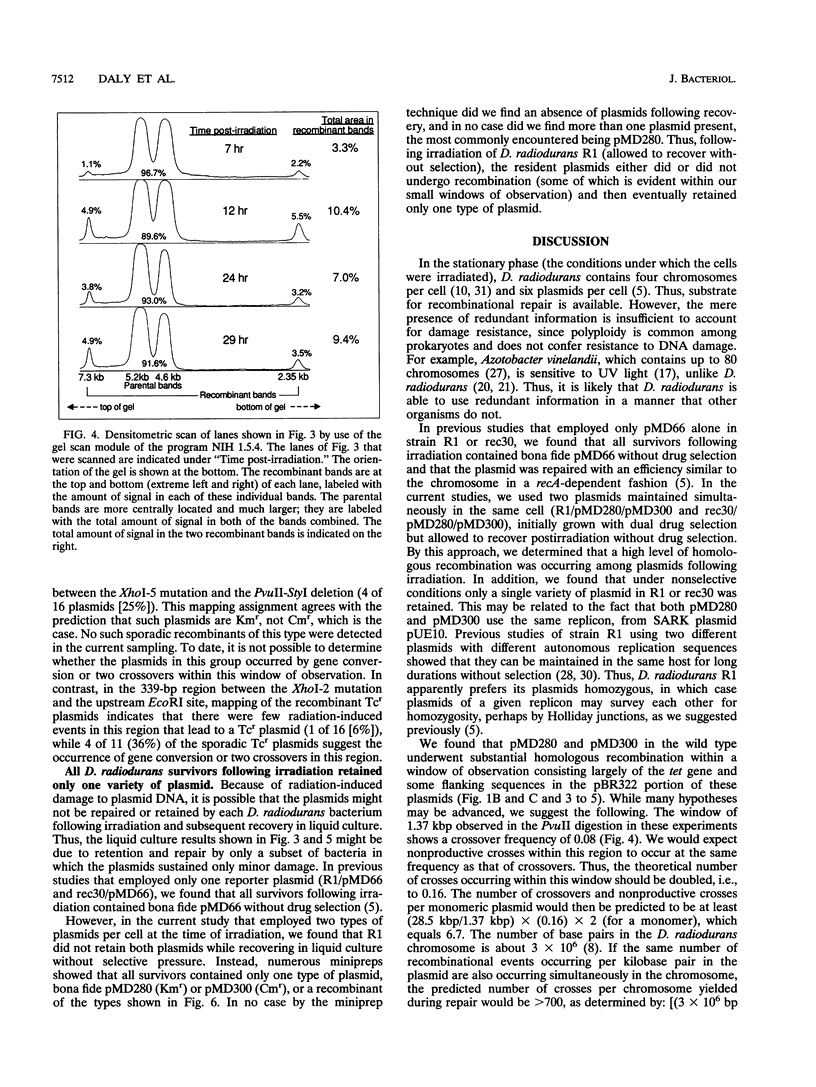
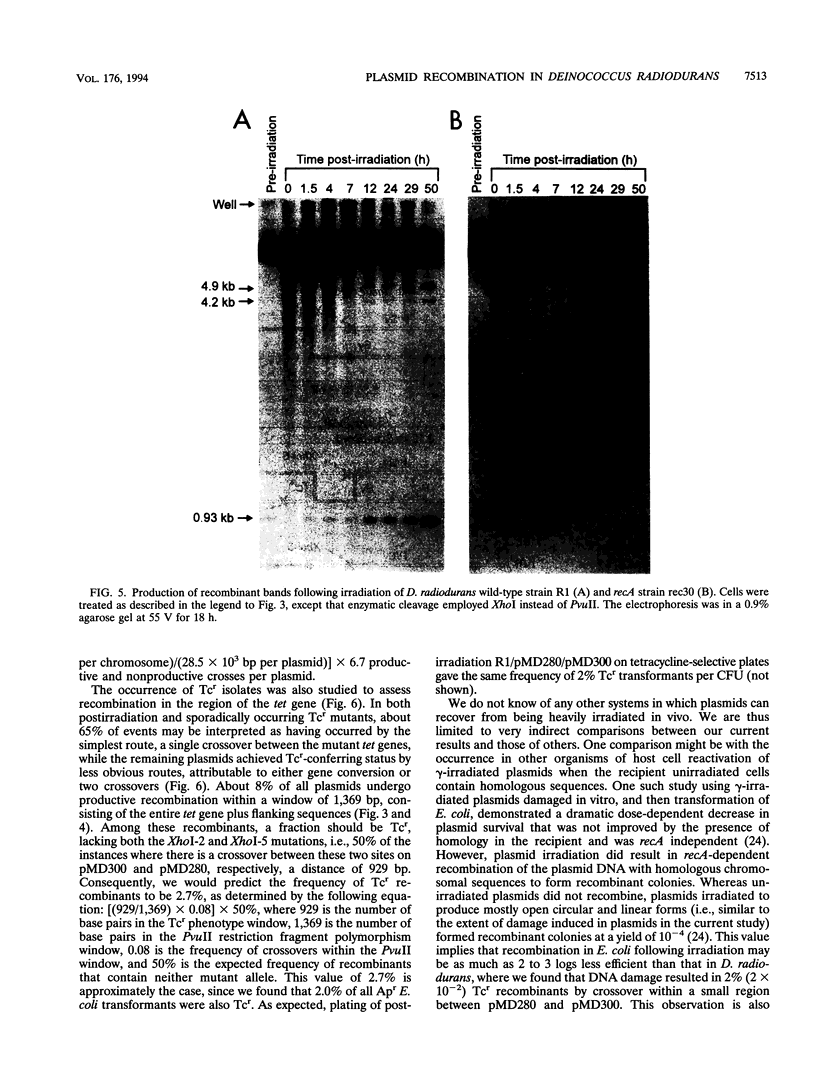
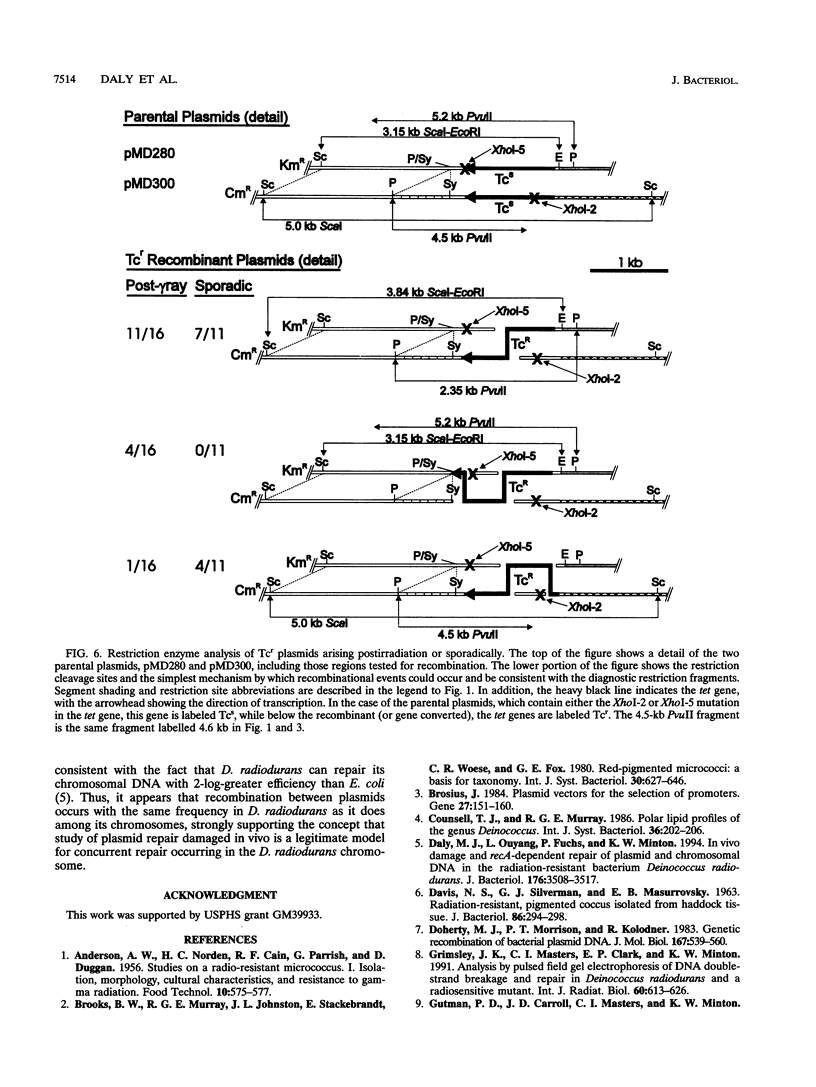
Deinococcus radiodurans R1 and other members of the eubacterial family Deinococcaceae are extremely resistant to ionizing radiation and many other agents that damage DNA. For example, after irradiation, D. radiodurans can repair > 100 DNA double-strand breaks per chromosome without lethality or mutagenesis, while most other organisms can survive no more than 2 or 3 double-strand breaks. The unusual resistance of D. radiodurans is recA dependent, but the repair pathway(s) is not understood. Recently, we described how a plasmid present in D. radiodurans (plasmid copy number, approximately 6 per cell; chromosome copy number, approximately 4 per cell) during high-dose irradiation undergoes extreme damage like the chromosome and is retained by the cell without selection and fully repaired with the same efficiency as the chromosome. In the current work, we have investigated the repair of two similar plasmids within the same cell. These two plasmids were designed to provide both restriction fragment polymorphisms and a drug selection indicator of recombination. This study presents a novel system of analysis of in vivo damage and recombinational repair, exploiting the unique ability of D. radiodurans to survive extraordinarily high levels of DNA damage. We report that homologous recombination among plasmids following irradiation is extensive. For example, 2% of Tcs plasmids become Tcr as a result of productive recombination within a 929-bp region of the plasmids after repair. Our results suggest that each plasmid may participate in as many as 6.7 recombinational events during repair, a value that extrapolates to > 700 events per chromosome undergoing repair simultaneously. These results indicate that the study of plasmid recombination within D. radiodurans may serve as an accurate model system for simultaneously occurring repair in the chromosome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- DAVIS N. S., SILVERMAN G. J., MASUROVSKY E. B. RADIATION-RESISTANT, PIGMENTED COCCUS ISOLATED FROM HADDOCK TISSUE. J Bacteriol. 1963 Aug;86:294–298. doi: 10.1128/jb.86.2.294-298.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
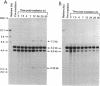
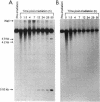
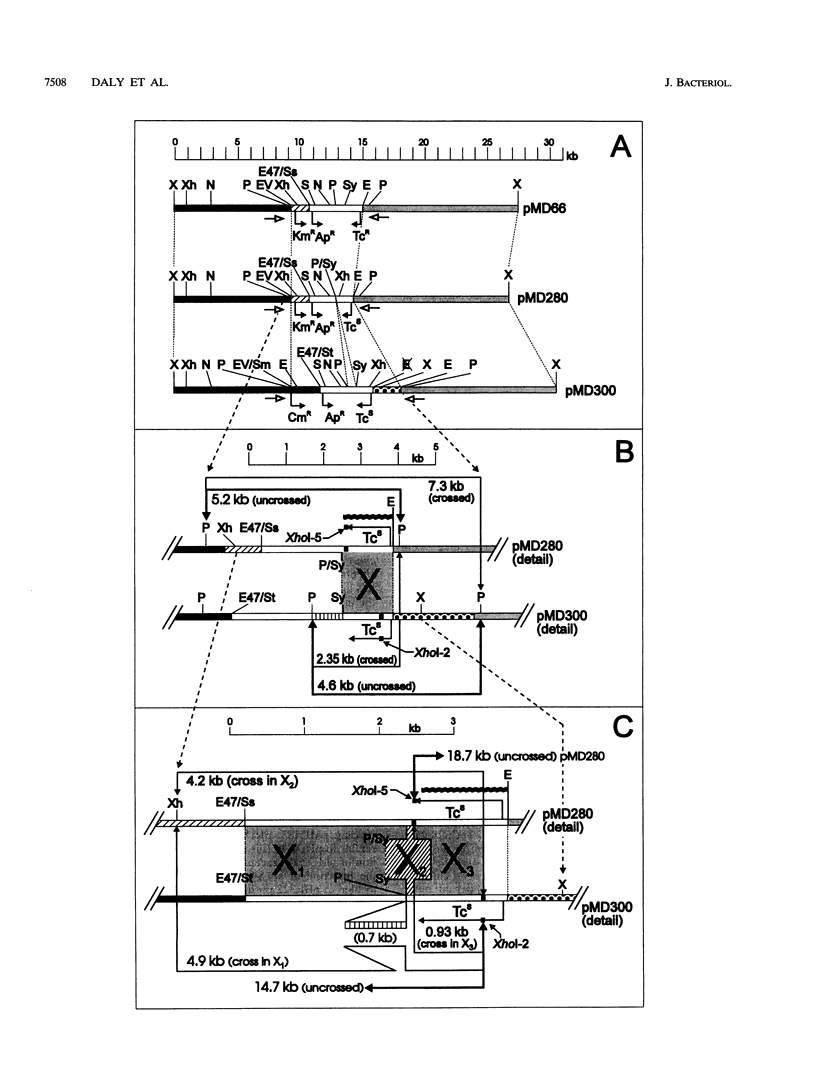
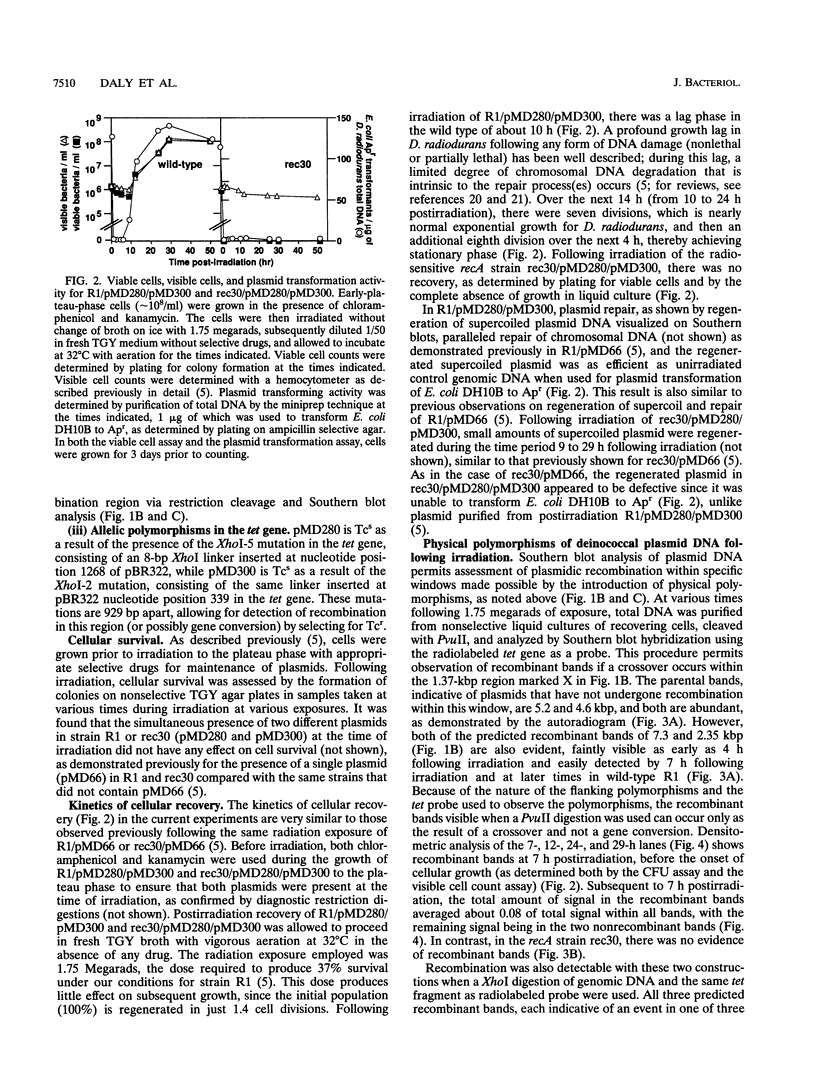
- Daly M. J., Ouyang L., Fuchs P., Minton K. W. In vivo damage and recA-dependent repair of plasmid and chromosomal DNA in the radiation-resistant bacterium Deinococcus radiodurans. J Bacteriol. 1994 Jun;176(12):3508–3517. doi: 10.1128/jb.176.12.3508-3517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M. J., Morrison P. T., Kolodner R. Genetic recombination of bacterial plasmid DNA. Physical and genetic analysis of the products of plasmid recombination in Escherichia coli. J Mol Biol. 1983 Jul 5;167(3):539–560. doi: 10.1016/s0022-2836(83)80097-7. [DOI] [PubMed] [Google Scholar]
- Grimsley J. K., Masters C. I., Clark E. P., Minton K. W. Analysis by pulsed-field gel electrophoresis of DNA double-strand breakage and repair in Deinococcus radiodurans and a radiosensitive mutant. Int J Radiat Biol. 1991 Oct;60(4):613–626. doi: 10.1080/09553009114552441. [DOI] [PubMed] [Google Scholar]
- Hansen M. T. Multiplicity of genome equivalents in the radiation-resistant bacterium Micrococcus radiodurans. J Bacteriol. 1978 Apr;134(1):71–75. doi: 10.1128/jb.134.1.71-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobatake M., Tanabe S., Hasegawa S. Nouveau Micrococcus radiorésistant à pigment rouge, isolé de fèces de Lama glama, et son utilisation comme indicateur microbiologique de la radiostérilisation. C R Seances Soc Biol Fil. 1973;167(10):1506–1510. [PubMed] [Google Scholar]
- Krasin F., Hutchinson F. Repair of DNA double-strand breaks in Escherichia coli, which requires recA function and the presence of a duplicate genome. J Mol Biol. 1977 Oct 15;116(1):81–98. doi: 10.1016/0022-2836(77)90120-6. [DOI] [PubMed] [Google Scholar]
- Lennon E., Minton K. W. Gene fusions with lacZ by duplication insertion in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1990 Jun;172(6):2955–2961. doi: 10.1128/jb.172.6.2955-2961.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. F. Studies on a radio-resistant coccus isolated from Bombay duck (Harpodon nehereus). J Gen Microbiol. 1971 Apr;66(1):29–35. doi: 10.1099/00221287-66-1-29. [DOI] [PubMed] [Google Scholar]
- Majumdar S., Chandra A. K. UV-repair and mutagenesis in Azotobacter vinelandii. I. Repair of UV-induced damages. Zentralbl Mikrobiol. 1985;140(3):247–254. [PubMed] [Google Scholar]
- Masters C. I., Minton K. W. Promoter probe and shuttle plasmids for Deinococcus radiodurans. Plasmid. 1992 Nov;28(3):258–261. doi: 10.1016/0147-619x(92)90057-h. [DOI] [PubMed] [Google Scholar]
- Masters C. I., Smith M. D., Gutman P. D., Minton K. W. Heterozygosity and instability of amplified chromosomal insertions in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1991 Oct;173(19):6110–6117. doi: 10.1128/jb.173.19.6110-6117.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton K. W. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol Microbiol. 1994 Jul;13(1):9–15. doi: 10.1111/j.1365-2958.1994.tb00397.x. [DOI] [PubMed] [Google Scholar]
- Moseley B. E., Copland H. J. Isolation and properties of a recombination-deficient mutant of Micrococcus radiodurans. J Bacteriol. 1975 Feb;121(2):422–428. doi: 10.1128/jb.121.2.422-428.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley B. E., Setlow J. K. Transformation in Micrococcus radiodurans and the ultraviolet sensitivity of its transforming DNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):176–183. doi: 10.1073/pnas.61.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett J. S., Manzella J. M., Taylor W. D. Homologous recombination and mutagenesis of gamma-irradiated plasmid DNA in Escherichia coli host cells. Radiat Res. 1990 Oct;124(1):57–61. [PubMed] [Google Scholar]
- Murray K., Mundy A. R., Blackford H. N., Stephenson T. P. Transvesical phenolisation of the pelvic plexuses: a simple technique for the treatment of refractory detrusor instability and hyperreflexia. Urol Int. 1986;41(3):202–206. doi: 10.1159/000281198. [DOI] [PubMed] [Google Scholar]
- Nagpal P., Jafri S., Reddy M. A., Das H. K. Multiple chromosomes of Azotobacter vinelandii. J Bacteriol. 1989 Jun;171(6):3133–3138. doi: 10.1128/jb.171.6.3133-3138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Abrahamson R., Minton K. W. Shuttle plasmids constructed by the transformation of an Escherichia coli cloning vector into two Deinococcus radiodurans plasmids. Plasmid. 1989 Sep;22(2):132–142. doi: 10.1016/0147-619x(89)90022-x. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Lennon E., McNeil L. B., Minton K. W. Duplication insertion of drug resistance determinants in the radioresistant bacterium Deinococcus radiodurans. J Bacteriol. 1988 May;170(5):2126–2135. doi: 10.1128/jb.170.5.2126-2135.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. D., Masters C. I., Lennon E., McNeil L. B., Minton K. W. Gene expression in Deinococcus radiodurans. Gene. 1991 Feb 1;98(1):45–52. doi: 10.1016/0378-1119(91)90102-h. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Giovannoni S. J., Woese C. R. The Deinococcus-Thermus phylum and the effect of rRNA composition on phylogenetic tree construction. Syst Appl Microbiol. 1989;11:128–134. doi: 10.1016/s0723-2020(89)80051-7. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]