Abstract
Proliferating cells in the subventricular zone (SVZ) of adult rat brain could provide a source of cells for repair attempts during degenerative diseases. However, very few reports dealt with the spontaneous regulation of this cell population during experimental conditions. In this paper, we describe an increase in the proliferation activity in the SVZ during experimental allergic encephalomyelitis, a demyelinating disease widely used as an experimental model for human multiple sclerosis. Moreover, p75LNGFR-immunoreactive elements in the SVZ were larger in experimental allergic encephalomyelitis compared with control groups, and they also showed multiple and branched elongations. Finally, a selective uptake of 125I-nerve growth factor was observed in the SVZ in neonatal rats, and positive elements migrated in the corpus callosum within a few days. These data indicate that cell populations in the SVZ are regulated during inflammatory conditions and degenerative diseases involving oligodendrocytes and neurotrophins, including nerve growth factor, could participate in these phenomena.
The repair capability of the nervous system is extremely low in adult life, in view of the postmitotic nature of neural and glial elements. This at least partially accounts for the devastating consequences of acute damage (i.e., stroke, trauma, etc.) and long-lasting neurodegenerative processes (i.e., Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, etc.). Experimental allergic encephalomyelitis (EAE) and multiple sclerosis are degenerative diseases characterized by the severe loss of oligodendrocytes and consequent demyelination, probably caused by inflammatory and autoimmune components. We previously have reported that nerve growth factor (NGF) increases in the cerebrospinal fluid of patients affected by multiple sclerosis (1) and also in brain areas of rats during EAE (2), where p75LNGFR immunoreactivity also is strongly up-regulated according to the severity of inflammatory cellular infiltrate (2). These and other data suggest a functional relation between the NGF level, p75LNGFR regulation, and the stage of the disease. In these conditions, NGF and related neurotrophins, such as other growth factors, are believed to play a major role in protection such as repair attempts. However, effective strategies to transfer successful experimental approaches aiming at manipulating endogenous levels of neurotrophins to stimulate protective and repair phenomena in humans are not still available.
The recent discovery of multipotent dividing, epidermal growth factor-sensitive cells in the subventricular zone (SVZ) of the mature central nervous system (CNS) in rodents and also in humans has offered a new potentiality for brain repair strategies. This large population of rapidly and constitutively proliferating cells gives rise not only to periglomerular neurons and interneurons in the olfactory bulb, but also to glial cells including oligodendrocytes (3, 4). In vivo (5) and in vitro (6) studies have indicated that growth factors, including epidermal growth factor (7), fibroblast growth factor (8, 9), cytokines (10), and neurotrophins (11) are involved in the differentiation, migration, and maturation of SVZ cells. However, very few studies suggest the possibility that these phenomena are spontaneously modulated by acute lesions of the mature CNS or during neurodegenerative diseases. In this paper we report that the mitotic index in the SVZ, as evaluated by using the proliferation-associated antigen Ki67, increases during EAE, where a phenotype regulation also apparently directed toward a rapid differentiation of p75LNGFR-expressing cells takes place. The eventual role of NGF in such processes is discussed.
MATERIALS AND METHODS
Animals.
Female, pathogen-free Lewis rats (Charles River Breeding Laboratories) were used. A group of rats was sensitized with a medium containing 29 mg of guinea pig spinal cord tissue in complete Freund’s adjuvant (CFA, Sigma) to which 2 mg/ml of heat-inactivated Mycobacterium (Difco H37Ra) was added. Sensitization was performed in both hind pads. A group of rats was injected in both hind pads with CFA. Uninjected rats were used as controls. Animals were killed 3, 7, 14, 20, and 75 days after treatment (n = 5 in each experimental group and for each time point). Rats were observed daily for signs of EAE and were scored as follows: 1) loss of tail tonicity, 2) weakness in one or both hind legs or middle ataxia, 3) severe ataxia or paralysis, and 4) severe hind leg paralysis accompanied by urinary incontinence. All animal protocols described here have been approved by our intramural committee and the animals were cared for in accordance with the guideline published in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunocytochemistry.
The day they were killed, rats were anesthetized and perfused through the ascending aorta with saline solution followed by 4% paraformaldehyde. Brains were removed, immersed for 24 hr in the same ice-cold fixative, and then rinsed for 48 hr in ice-cold 0.1 M Sorensen’s buffer containing 5% sucrose. Vibratome sections (thickness 50 μm) were immediately processed for the avidin-biotin procedure on free-floating sections. The sections were pretreated with H2O2 to quench endogenous peroxidase activity and then incubated with 1.5–2.0% normal serum, followed by overnight incubation at 4°C with the primary antisera diluted in PBS containing 0.3% Triton X-100 (vol/vol). The following antisera were used: mouse monoclonal anti-p75LNGFR-antibody, 1:5 to 1:125 (clone IgG192, generously supplied by E.M. Johnson, Washington University, St. Louis); rabbit polyclonal anti-trkA, 1:300 to 1:1,000 (Santa Cruz Biotechnology); rabbit polyclonal anti-Ki67, 1:1000 to 1:30,000 (NovoCastra, Newcastle, U.K.). Staining specificity was assessed by the in vitro overnight preincubation of the antiserum with the respective antigen (100 mg/ml). The peptide 763–777 adjacent to the carboxy terminus of the precursor form of Trk gp 140 of human origin was used for TrkA-antiserum preabsorption. This treatment prevented the staining. After the sections had been rinsed, they were incubated with biotinylated goat anti-mouse or anti-rabbit Ig (Dako) containing normal rat serum 1%, rinsed in PBS, and incubated by using streptavidin-biotinylated horseradish peroxidase complex (Amersham) 1:250. Alternatively, sections were developed by using the tyramide-intensification procedure (NEN, TSA-Indirect kit). Briefly, after incubation with the primary antiserum, sections were incubated with biotinylated anti-rabbit serum (Dako) followed by repeated washing and incubation with streptavidin-horseradish peroxidase. Biotinyl-tyramide then was applied to the section for 7 min. Diaminobenzidine was used to detect the immunocomplex. Sections (20 μm) also were stained for histological examination (Toluidine blue and hematoxy-eosin staining).
125I-NGF Incorporation.
125I-NGF was radioiodinated with Na125I (Amersham) by the chloramine-T procedure (28) and purified by Sephadex G-25 column chromatography. The specific activity was 1.0–1.5 Ci/mmol. 125I-NGF was injected by using a stereotaxic apparatus (Narishige, Tokyo) with the incisor bar 4–5 mm above the interaural line. Injection was made into the right lateral ventricle by using a 5-μl syringe with a 31-gauge needle (Hamilton). The needle was placed 1.5 mm lateral to the bregma and inserted to a depth of 3.4 mm. Preliminary injections with dye were made to verify the right coordinates. Injections of 5 μl were made over a 3-min period. The needle then was slowly withdrawn over an additional 3-min period. A total of six animals were injected with 125I-NGF. Twenty-four and 48 hr after injection, the animals were anesthetized and perfused transcardially with 100 ml of buffered saline followed by 250 ml of 4% paraformaldehyde in phosphate buffer 100 mM, pH 7.4. Then the brains were removed and postfixed for overnight in the same fixative. The brains subsequently were placed in a 30% buffered sucrose solution for 24 hr. Sections of brain (15 μm thickness) were cut and used for autoradiography. Briefly, slides were coated with nuclear tracking emulsion Ilford K2 emulsion (Ilford), developed using Kodak D19 developer after 1 month’s exposure and counterstained with Toluidine blue.
Histopathology.
Toluidine blue and hematoxylin-eosin staining were used for histological studies.
Statistical Analysis.
ANOVA and Dunnett’s test (vs. control uninjected rats) were used to compare data at each experimental time.
RESULTS
In this paper we have compared rats affected by EAE to adjuvant-injected and uninjected (i.e., control) ones. Histological and immunohistochemical observations were performed 7, 14, 21, and 75 days after active immunization of the animals. As already described (2), the most severe clinical indices of EAE were observed between 8 and 12 days, whereas the most severe histological indices of brain inflammation were described 21 days after immunization (presence of mononuclear and polynuclear cells in perivascular areas and into aggregates in the white and gray matter; presence of inducible nitric-oxide synthese-expressing macrophages; areas of necrosis and vascularization). Perivascular cellular infiltrates were still present 75 days after immunization.
p75LNGFR in Dividing Cells in the SVZ in Adult Rats.
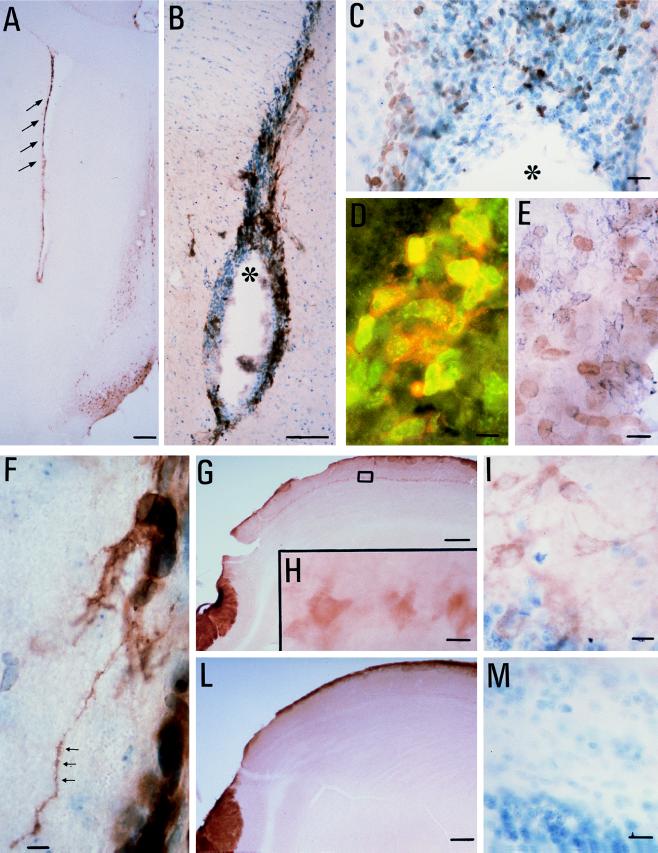
The SVZ extending from the lateral ventricle to the olfactory bulb expresses high levels of p75LNGFR immunoreactivity. The positivity is distributed along the external surface of cell bodies in many cellular elements, most of which are localized in the lateral SVZ and the dorso-lateral corner (Fig. 1 A and B). The positivity was absent in control slides incubated without the primary antiserum. p75LNGFR positivity covers approximately 20% of the SVZ, and the positive elements are mixed with the negative ones. Dividing elements were identified for the presence of Ki67 protein along the rostro-caudal extension of the SVZ to the olfactory bulb (Fig. 1C). Ki67 is a nuclear protein expressed by cycling cells during the late G1, S, G2, and M stages (12). Most of the dividing cells in adult CNS were localized in the SVZ, all over its rostrocaudal extension. Double-labeling experiments revealed that a percentage of dividing cells also expressed p75LNGFR immunoreactivity (Fig. 1D) without preferential localization of its coexistence along the rostrocaudal axis. We found a partial coexistence of Ki67 positivity with polysialylated-neural cell adhesion molecule positive elements, too (Fig. 1E).
Figure 1.
(A and B) p75LNGFR immunoreactivity is localized in the SVZ of the adult rat brain at rostrocaudal level 1.7 mm (refs. 13 and 14, tyramide-intensification procedure). The arrows point to positive staining in the SVZ, and the asterisk indicates the lateral ventricle. In the dorso-lateral corner of the lateral ventricle sampled at this level, numerous cycling, Ki67-positive cells (C, avidin-biotin technique; the asterisk indicates the ventricle), partially coexisting with p75LNGFR and neural cell adhesion molecule positivity are observed. (D) Double-labeling immunofluorescence, in which the green fluorescence refers to Ki67-positive nuclei and red fluorescence refers to p75LNGFR. Fluoresceine isothiocyanate- and rhodamine-conjugated Ig were from Dako. (E) Double-labeling avidin-biotin technique. The brown diaminobenzidine product refers to Ki67 positivity, whereas the blue chloronaphthol product refers to neural cell adhesion molecule positivity. Fibers departing from p75LNGF-positive elements are observed in the SVZ of the lateral ventricle during EAE (F). TrkA positivity also was observed in the mitral cells in the olfactory bulb (G: low-power magnification, 2×; H: higher magnification, 40×, referring to the square area indicated in G; I: toluidine, controstained TrkA-positive section). TrkA positivity was not observed in the mitral cells of control rats (L and M). [Bars = 200 μm (A, G, and L), 50 μm (B), 25 μm (C, F, H, I, and M), and 15 μm (D and E).]
Proliferative Index in EAE.
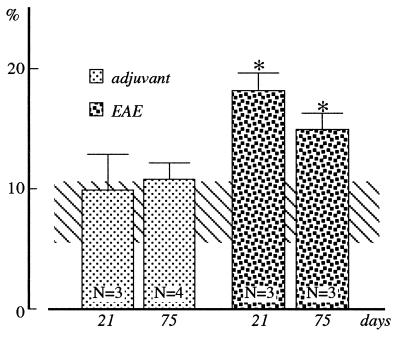
The percentage area covered by Ki67 positivity was used to calculate the “proliferative index” in the SVZ. Considering the rostro-to-caudal decrease in the proliferation rate, we analyzed the “dorso-lateral corner” of the lateral ventricle at level 1.7 mm from the bregma according to Paxinos and Watson stereotaxic atlas of the brain (13) (see also ref. 14), where a large population of proliferating cells has been described. Five nonconsecutive sections were analyzed in each animal, and the mean value then was used for the statistical analysis. Using [3H]thymidine incorporation, which identifies cells in S stage of the cell cycle, the proportion of labeled cells in this area is 2.5% (15, 16). In control rats, we found about 8% of Ki67-positive cells in the total SVZ cell population (Fig. 2). In other proliferating cell populations, like epithelial cells or different tumor cells, a similar ratio between markers for the S phase alone (i.e., [3H]thymidine incorporation), and proliferation-associated markers, including Ki67, has been described (17). Evaluation of Ki67 immunoreactivity revealed a doubling of the mitotic index during EAE, compared with both control and adjuvant-injected rats (Fig. 2). The elevation was still present 75 days after immunization.
Figure 2.
Cycling cells in the SVZ in control (horizontal bar), adjuvant-injected, and EAE rats. The results are expressed as percentage area covered by the Ki67 positivity in the dorsolateral corner of the lateral ventricle. Statistical analysis: ANOVA and Dunnett’s test, ∗, P < 0.05.
Regulation of p75LNGFR-Positive Cells in SVZ During EAE.
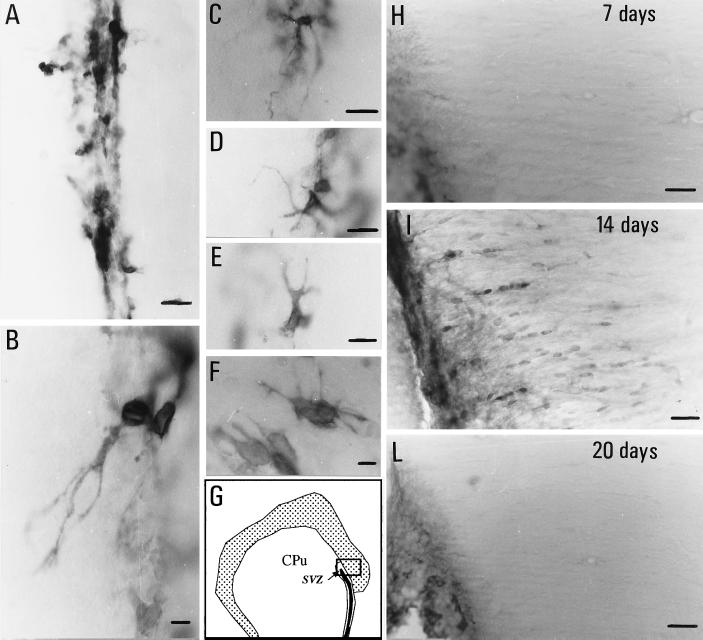
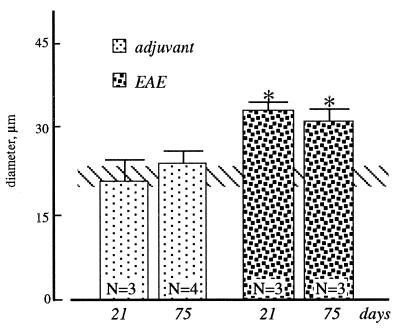
p75LNGFR positivity in the SVZ and adjacent white and gray matter is modified during EAE. In particular, chains of small, ovoidal positive cells were observed 14 days after immunization, departing from the border of the ventricle toward the white matter of the corpus callosum (Fig. 3I). This was not observed in adjuvant-injected rats or at different times (7, 20, or 75 days) after immunization (Fig. 3 H and L). The area covered by p75LNGFR-positive elements in the SVZ in EAE animals, as evaluated 21 and 75 days after immunization, exceeds that found in adjuvant-injected and control rats. This could be partially caused by the increase in the mean area of the positive elements observed during EAE (Fig. 4). Moreover, morphological changes also were observed in a part of p75LNGFR-positive elements in EAE rats (Fig. 3 A–F). Many elements along the ventricles and in the dorso-lateral corner showed elongations. These fibers were multiple, short, and branched (Fig. 3F), but also long elongations arise from p75LNGFR-positive elements, departing from the cell body through a single “cone” and then dividing into multiple branches (Figs. 1F and 3 B–E). In some cases a well-defined direction toward the lateral, surrounding cerebral tissue was identified, whereas in other elements an irregular sprouting without defined directions or targets was observed.
Figure 3.
p75LNGFR immunoreactivity in the SVZ of control (A) and EAE (B–F) rats, 21 days after immunization. Micrographs B–F illustrate the diffuse and branched fiber outgrowth observed during EAE. G shows the area sampled by micrographs H–L. Migrograph I shows p75LNGFR-positive cells in the corpus callosum 14 days after immunization, which were not observed at other time-points during EAE nor in control rats. CPu, caudato-putamen nucleus. (Bars = 25 μm.)
Figure 4.
Mean diameter of p75LNGFR-positive cells in the SVZ in control (horizontal bar), adjuvant-injected, and EAE rats. Cells were sampled in the dorsolateral corner of the lateral ventricle. Statistical analysis: ANOVA and Dunnett’s test, ∗, P < 0.05.
125I-NGF Uptake by the SVZ During Brain Maturation and trkA-Regulation in the Olfactory Bulb During EAE.
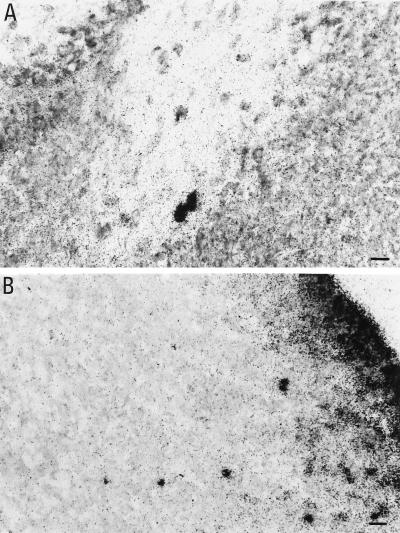
Searching for information as to whether NGF may be a potential ligand for p75LNGFR in the SVZ, radiolabeled NGF was injected in the lateral ventricles of developing rats and the distribution of NGF-positive cells in experimental brains was examined after survival time of 24, 48, and 72 hr. Virtually all pups (n = 4) that received 125I-NGF injection in the lateral ventricle displayed a stream of cells extending from the injection site to the olfactory bulb, as shown in Fig. 5. Twenty-four hours after injection, very few NGF-positive cells had begun to migrate toward the parenchyma (Fig. 5B). For example, the mean number of NGF-labeled cells observed in the caudate nucleus close to the ventricle (200 μm from the lateral ventricle) was 80 ± 20 and it decreased to 20 ± 8 48 hr after injection. Moreover, the mean number of labeled cells observed 1,000 μm toward the ventricle was 18 ± 7 at 24 hr, and it increases to 53 ± 11 at 48 hr (unpublished data). At this time numerous cells were distributed below the forming corpus callosum, constituting the vertical limb of this structure (Fig. 5A).
Figure 5.
125I-NGF uptake in the SVZ (B) and the corpus callosum (A) of neonatal rats. (Bar = 20 μm.)
To explore a possible involvement of NGF in the regulation of SVZ migration, we also analyzed the regulation of trkA expression during EAE by immunocytochemistry in the SVZ and the olfactory bulb (Fig. 1 G–M). Although no trkA-positive signal was detectable in the SVZ when using the Santa Cruz antibody (this also was confirmed by in situ hybridization experiments using an oligoprobe), an up-regulation of trkA immunoreactivity was found in the olfactory bulb during EAE. A clear positivity was in fact observed in the mitral cell layer of the olfactory bulb (Fig. 1 G–I) that was not found in control rats (Fig. 1 L and M). Notably, no p75LNGFR-positive staining was observed in the mitral cell during EAE.
DISCUSSION
In this paper we have provided a clear description of p75LNGFR immunoreactivity in the SVZ and its partial coexistence with the proliferating antigen Ki67. Ki67 also is coexpressed in a percentage of SVZ cells that are positive for the neural cell adhesion molecule (NCAM). We also have described the early morphological differentiation, already in the SVZ, of some p75LNGFR-positive cells during EAE, which show a considerable fiber outgrowth elongating into the surrounding brain areas. Finally, we have shown that the mitotic index increases in the SVZ during EAE, providing evidence that the dividing cells in adult CNS may be influenced during degenerative diseases.
After the recent discovery of multipotential precursor cells in the adult CNS (18), most of the experimental effort has been devoted to understanding the basal state and the lineage potentiality of these cells (9). It has been demonstrated that these cycling cells may not only die but also generate glia, or neurons, or both (14, 19–21), and the role of trophic factors in division, differentiation, migration, and maturation of precursor cells in the adult CNS is under extensive investigation. However, although the use of precursor cells in repair strategies in the mature CNS is an extremely attractive perspective, very little work has been directed to exploring the ability of the SVZ population to respond to acute or chronic injuries. Two papers have indicated that acute neural lesions can influence the proliferation rate in the SVZ in adult rats. Weinstein and coworkers (15) have described the increased proliferation of precursor cells in the adult brain after fimbria fornix lesion, and Szele and Chesselet (22) have been able to demonstrate that cortical lesions induce an increase in cell numbers and phenotype regulation in the SVZ of adult rats. Recently it also has been suggested that cells of the adult mouse SVZ proliferate, migrate, and differentiate into oligodendrocytes in response to chemical-induced demyelination (23).
Here, we have shown that a chronic disease affecting oligodendrocytes also increases the proliferating rate in the SVZ. The fate of the daughter cells in this particular condition, too, is still under debate. They can be incorporated as proliferating cells in the SVZ, they can die, or they can migrate and differentiate in glial or neural lineage. The partial coexistence of proliferating antigen Ki67 and p75LNGFR and the phenotype regulation of p75LNGFR-expressing cells suggest different possibilities, also in view of the particular microenvironment in the SVZ during EAE. The breakdown of the blood-brain barrier in fact, exposes the SVZ population to unique humoral stimulation, comprising neurotrophins, cytokines, peptides, and peripheral hormones (24). The p75LNGFR has different potential ligands and can induce different cellular responses. NGF binding to p75LNGFR affects neuronal survival during development (25), and p75LNGFR also acts as a trkA regulator in a neuronal progenitor cell line to acquire a mature neuronal morphology more rapidly (26). Although we have been unable to identify trkA receptor in the SVZ either in control or EAE animals, a clear up-regulation of this receptor has been observed during EAE in the olfactory bulb, which is a target area of migrating SVZ cells.
An attractive hypothesis is that precursors in the SVZ can require neurotrophins for migration and differentiation. In view of the severe damage to brain-blood barrier, the SVZ is exposed to high concentrations of NGF during EAE, according to a time course that corresponds to the presence of distinct chains of p75LNGFR cells directed toward the white matter of corpus callosum. p75LNGFR could be expressed by differentiating cells under influence of increased levels of NGF itself, or by the stimulating action of other cytokines, including tumor necrosis factor (27). Here we further confirm a selective uptake of 125I-NGF by the SVZ cells in neonatal/postnatal rat brains, at least in some stages of maturation. In a previous study, it was demonstrated that NGF induces proliferation and differentiation of tanicytes in tadpole brains, suggesting that multipotent cells localized along the brain ventricle are highly receptive to the action of NGF (28). Moreover, NGF regulates proliferation and differentiation of neuronal stem cells that have been previously exposed to basic fibroblast growth factor (29). Evidence that NGF might be involved in this process is suggested by other studies. Migrating Schwann cells in developing or regenerating peripheral nerves are known to express extremely high levels of NGF and p75LNGFR (30), suggesting that NGF also leads to specific events such as migration (31). The presence of p75LNGFR with high levels of NGF has been associated with developmental processes that affect morphogenesis and cell differentiation (32). Moreover, cytokines have been proved to cause the elaboration of oligodendroglial progenitor and postmitotic oligodendrocytes in a cellular system obtained from the SVZ (10).
In vitro studies indicate that spheres obtained by embrional striatum differentiate into neuronal and oligodendroglial cells in the presence of neurotrophic factors (33). NGF results in bipolar neurons, whereas oligodendrocytes appear after ciliary neurotrophic factor and neurotrophin-3 treatment. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat SVZ (11), and it enhances differentiation of CNS stem-derived neuronal precursors, leading to multipolar neuronal cells (34). It has been suggested that it also may act as a permissive factor for neuronal recruitment in adulthood (11).
However, we should mention that recent evidence suggests that p75LNGFR also could participate in the regulation of apoptotic pathways, e.g., in oligodendrocytes (35), when trkA is absent and in relation to NGF availability. In view of this, the increased availability of NGF during EAE may activate the NF-κB intracellular pathway potentiating cell survival (36). It has been demonstrated that NGF causes translocation of NF-κB to the nucleus of Schwann cells (37), and this is absent in p75LNGFR−/− mice. On the contrary, glioblastoma cells, which express p75LNGFR but not trkA receptors in vitro, showed a reduced growth rate and formed cytoplasmic processes on treatment with NGF (38).
In conclusion, in this paper we have described an increased mitotic index and a regulation of phenotype expression of dividing cells in the SVZ through p75LNGFR in the adult CNS during EAE. The in vivo phenotype regulation of these cells during a disease characterized by degenerative processes involving glial and neural cells also suggests the possibility of exogenous stimulation for commitment of this population. Moreover, we cannot exclude that defined conditions, spontaneously or therapeutically induced, also may recruit quiescent stem cells in other areas of the CNS during EAE.
Acknowledgments
We are grateful to Prof. Rita Levi-Montalcini for her encouragement and helpful discussion during this study. We wish to thank Prof. S. Garbisa, University of Padova, for suggestions in the study of cycling cells. This work was supported by Associazione Italiana Sclerosi Multipla (L.C.) and Consiglio Nazionale delle Ricerche, Progetto Neuroscienze, Fattori trofici, recettori e rigenerazione del sistema nervoso centrale (R.L.M. and L.A.).
ABBREVIATIONS
- SVZ
subventricular zone
- EAE
experimental allergic encephalomyelitis
- NGF
nerve growth factor
- CNS
central nervous system
References
- 1.Bracci-Laudiero L, Aloe L, Levi-Montalcini R, Buttinelli C, Schilter D, Gillessen S, Otten U. Neurosci Lett. 1992;147:9–12. doi: 10.1016/0304-3940(92)90762-v. [DOI] [PubMed] [Google Scholar]
- 2.Calzà L, Giardino L, Pozza M, Micera A, Aloe L. Proc Natl Acad Sci USA. 1997;94:3368–3373. doi: 10.1073/pnas.94.7.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levison S W, Goldman J E. Neuron. 1993;10:201–212. doi: 10.1016/0896-6273(93)90311-e. [DOI] [PubMed] [Google Scholar]
- 4.Steindler D A, Kadrie T, Fillmore H, Thomas L B. Prog Brain Res. 1996;108:349–363. [PubMed] [Google Scholar]
- 5.Kuhn H G, Winkler J, Kempermann G, Thal L J, Gage F H. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage F H, Jasodhara R, Fisher L J. Annu Rev Neurosci. 1995;18:159–192. doi: 10.1146/annurev.ne.18.030195.001111. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds B A, Weiss S. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 8.Gritti A, Parati E A, Cova L, Frolichstal P, Galli R, Wanke E, Faravelli L, Morassutti D J, Roisen F, Nickel D D, Vescovi A L. J Neurosci. 1996;16:1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss S, Reynolds B A, Vescovi A L, Morshead C, Craig C G, van der Kooy D. Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 10.Mehler M F, Marmur R, Gross R, Mabie P C, Zang Z, Papavasiliou A, Kessler J A. Int J Dev Neurosci. 1995;13:213–240. doi: 10.1016/0736-5748(94)00060-g. [DOI] [PubMed] [Google Scholar]
- 11.Kirschenbaum B, Goldman S A. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hofstadter F, Knuchel R, Ruschoff J. Virchows Arch. 1995;427:323–341. doi: 10.1007/BF00203402. [DOI] [PubMed] [Google Scholar]
- 13.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 14.Luskin M B. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein D E, Burrola P, Kilpatrick T J. Brain Res. 1996;743:11–16. doi: 10.1016/s0006-8993(96)00979-1. [DOI] [PubMed] [Google Scholar]
- 16.Morshead C M, Reynolds B A, Craig C G, McBurney M W, Staines W A, Morassutti D, Weiss S, van der Kooy D. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 17.Smith M D, Healy E, Thompson V, Morley A, Rees J L. Br J Dermatol. 1995;132:359–366. doi: 10.1111/j.1365-2133.1995.tb08668.x. [DOI] [PubMed] [Google Scholar]
- 18.Privat A, Leblond C P. J Comp Neurol. 1972;146:227–302. doi: 10.1002/cne.901460302. [DOI] [PubMed] [Google Scholar]
- 19.Frederiksen K, McKay R D G. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lois C, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zerlin M, Levison S W, Goldman J E. J Neurosci. 1995;15:7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szele F G, Chesselet M-F. J Comp Neurol. 1996;368:439–454. doi: 10.1002/(SICI)1096-9861(19960506)368:3<439::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Lachapelle F, Nait-Oumesmar B, Decker L, Peterson A, Baron-Van Evercooren A. Soc Neurosci Abstr. 1997;23:662.6. [Google Scholar]
- 24.Hawkins C P, Mackenzie F, Tofts P, du Boulay E P G H, McDonald W I. Brain. 1991;114:801–810. doi: 10.1093/brain/114.2.801. [DOI] [PubMed] [Google Scholar]
- 25.Ryden M, Hempstead B, Ibanez C F. J Biol Chem. 1997;272:16322–16328. doi: 10.1074/jbc.272.26.16322. [DOI] [PubMed] [Google Scholar]
- 26.Verdi J M, Anderson D J. Neuron. 1994;13:1359–1372. doi: 10.1016/0896-6273(94)90421-9. [DOI] [PubMed] [Google Scholar]
- 27.Pan W, Banks W A, Kennedy M K, Gutierrez E G, Kastin A J. Am J Physiol. 1996;271:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. [DOI] [PubMed] [Google Scholar]
- 28.Levi-Montalcini R, Aloe L. Proc Natl Acad Sci USA. 1985;82:7111–7115. doi: 10.1073/pnas.82.20.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cattaneo E, McKay R. Nature (London) 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 30.You S, Petrov T, Chung P H, Gordon T. Glia. 1997;20:87–100. doi: 10.1002/(sici)1098-1136(199706)20:2<87::aid-glia1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Singh N, Birdi T J, Antia N H. Neuropathol Appl Neurobiol. 1997;23:59–67. [PubMed] [Google Scholar]
- 32.Wheeler E F, Bothwell M. J Neurosci. 1992;12:930–945. doi: 10.1523/JNEUROSCI.12-03-00930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachyankar M B, Condon P J, Quesenberry P J, Litofsky N S, Recht L D, Ross A H. Exp Neurol. 1997;144:350–360. doi: 10.1006/exnr.1997.6434. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed S, Reynolds B A, Weiss S. J Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casaccia-Bonnefil P, Carter B D, Dobrowsky R T, Chao M V. Nature (London) 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- 36.Dechant G, Barde Y-A. Curr Opin Neurobiol. 1997;7:413–418. doi: 10.1016/s0959-4388(97)80071-2. [DOI] [PubMed] [Google Scholar]
- 37.Carter B D, Kaltschmidt C, Kaltschmidt B, Offenhauser N, Bohm-Matthaei R, Baeuerle P A, Barde Y-A. Science. 1996;272:542–545. doi: 10.1126/science.272.5261.542. [DOI] [PubMed] [Google Scholar]
- 38.Matsushima H, Bogenmann E. Mol Cell Biol. 1993;13:7447–7456. doi: 10.1128/mcb.13.12.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]