Abstract
In Vibrio fischeri, the synthesis of N-3-oxohexanoyl-L-homoserine lactone, the autoinducer for population density-responsive induction of the luminescence operon (the lux operon, luxICDABEG), is dependent on the autoinducer synthase gene luxI. Gene replacement mutants of V. fischeri defective in luxI, which had been expected to produce no autoinducer, nonetheless exhibited lux operon transcriptional activation. Mutants released into the medium a compound that, like N-3-oxohexanoyl-L-homoserine lactone, activated expression of the lux system in a dose-dependent manner and was both extractable with ethyl acetate and labile to base. The luxI-independent compound, also like N-3-oxohexanoyl-L-homoserine lactone, was produced by V. fischeri cells in a regulated, population density-responsive manner and required the transcriptional activator LuxR for activity in the lux system. The luxI-independent compound was identified as N-octanoyl-L-homoserine lactone by coelution with the synthetic compound in reversed-phase high-pressure liquid chromatography, by derivatization treatment with 2,4-dinitrophenylhydrazine, by mass spectrometry, and by nuclear magnetic resonance spectroscopy. A locus, ain, necessary and sufficient for Escherichia coli to synthesize N-octanoyl-L-homoserine lactone was cloned from the V. fischeri genome and found to be distinct from luxI by restriction mapping and Southern hybridization. N-Octanoyl-L-homoserine lactone and ain constitute a second, novel autoinduction system for population density-responsive signalling and regulation of lux gene expression, and possibly other genes, in V. fischeri. A third V. fischeri autoinducer, N-hexanoyl-L-homoserine lactone, dependent on luxI for its synthesis, was also identified. The presence of multiple chemically and genetically distinct but cross-acting autoinduction systems in V. fischeri indicates unexpected complexity for autoinduction as a regulatory mechanism in this bacterium.
Full text
PDF

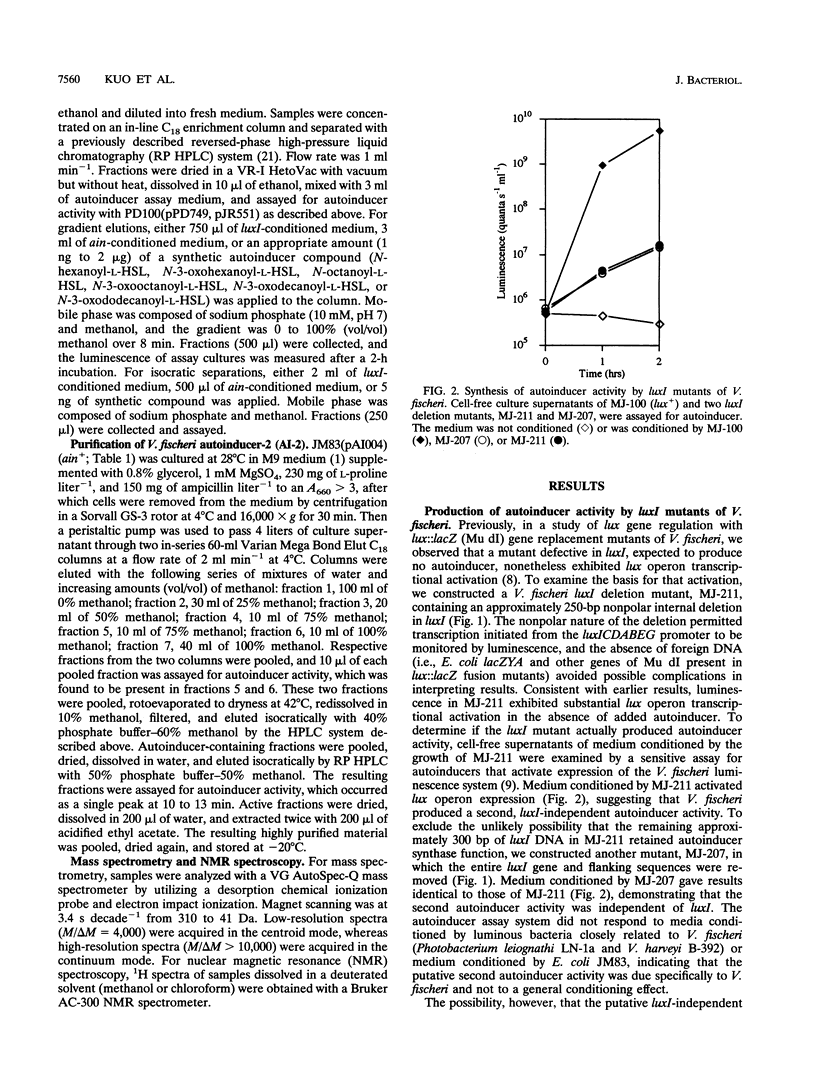
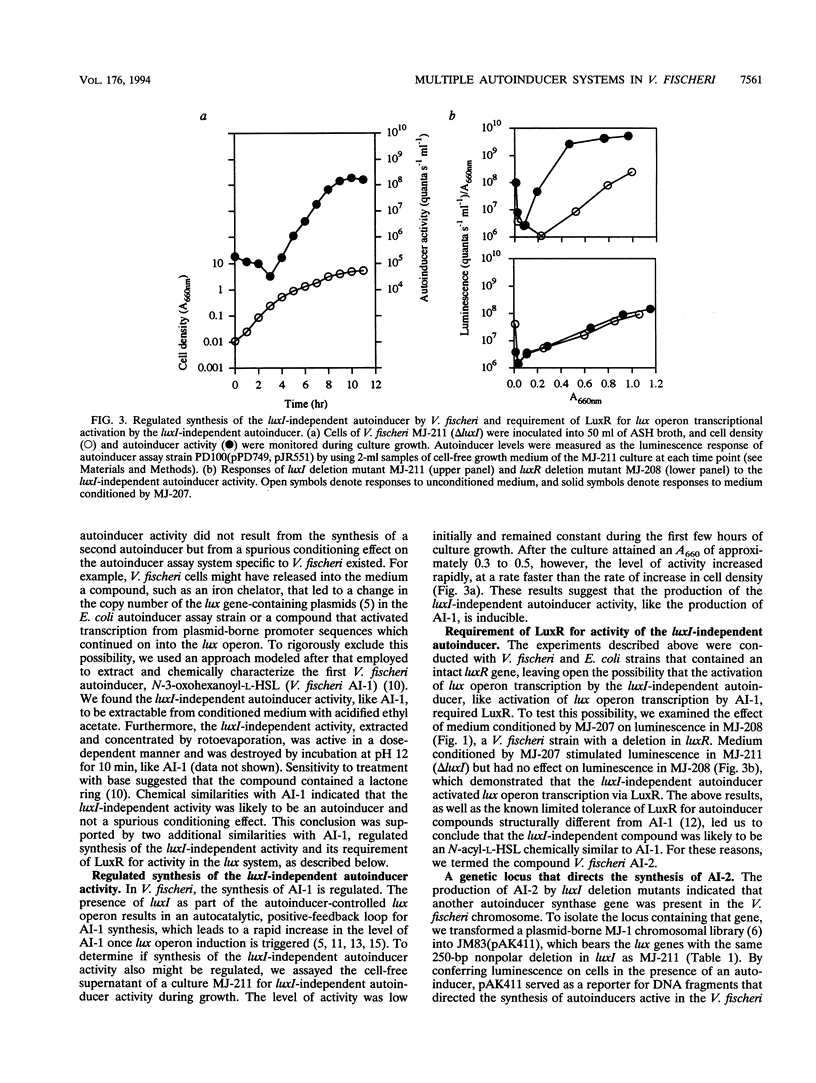
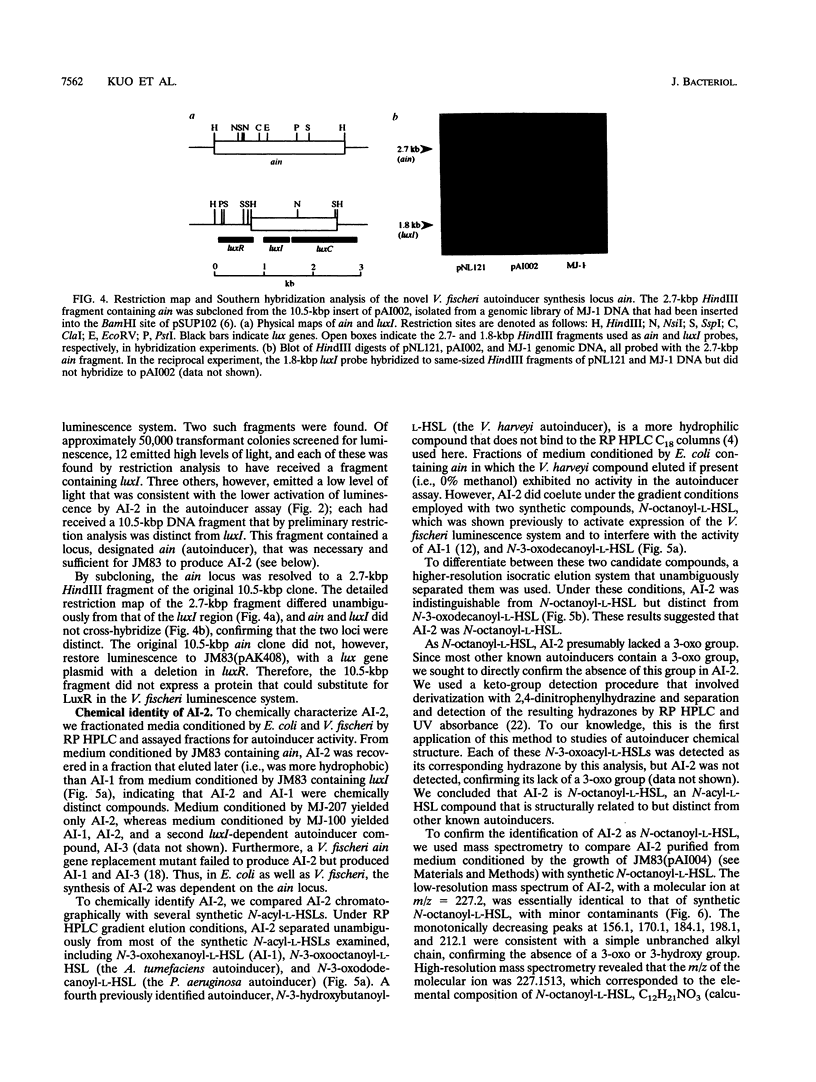


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainton N. J., Stead P., Chhabra S. R., Bycroft B. W., Salmond G. P., Stewart G. S., Williams P. N-(3-oxohexanoyl)-L-homoserine lactone regulates carbapenem antibiotic production in Erwinia carotovora. Biochem J. 1992 Dec 15;288(Pt 3):997–1004. doi: 10.1042/bj2880997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassler B. L., Wright M., Showalter R. E., Silverman M. R. Intercellular signalling in Vibrio harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol. 1993 Aug;9(4):773–786. doi: 10.1111/j.1365-2958.1993.tb01737.x. [DOI] [PubMed] [Google Scholar]
- Cao J. G., Meighen E. A. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J Biol Chem. 1989 Dec 25;264(36):21670–21676. [PubMed] [Google Scholar]
- Dunlap P. V., Callahan S. M. Characterization of a periplasmic 3':5'-cyclic nucleotide phosphodiesterase gene, cpdP, from the marine symbiotic bacterium Vibrio fischeri. J Bacteriol. 1993 Aug;175(15):4615–4624. doi: 10.1128/jb.175.15.4615-4624.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-luxR protein regulatory circuit. J Bacteriol. 1988 Sep;170(9):4040–4046. doi: 10.1128/jb.170.9.4040-4046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Kuo A. Cell density-dependent modulation of the Vibrio fischeri luminescence system in the absence of autoinducer and LuxR protein. J Bacteriol. 1992 Apr;174(8):2440–2448. doi: 10.1128/jb.174.8.2440-2448.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V. Mechanism for iron control of the Vibrio fischeri luminescence system: involvement of cyclic AMP and cyclic AMP receptor protein and modulation of DNA level. J Biolumin Chemilumin. 1992 Jul;7(3):203–214. doi: 10.1002/bio.1170070307. [DOI] [PubMed] [Google Scholar]
- Dunlap P. V., Ray J. M. Requirement for autoinducer in transcriptional negative autoregulation of the Vibrio fischeri luxR gene in Escherichia coli. J Bacteriol. 1989 Jun;171(6):3549–3552. doi: 10.1128/jb.171.6.3549-3552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua W. C., Winans S. C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994 May;176(10):2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambello M. J., Iglewski B. H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991 May;173(9):3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman G. W., Kolter R. Sensing starvation: a homoserine lactone--dependent signaling pathway in Escherichia coli. Science. 1994 Jul 22;265(5171):537–539. doi: 10.1126/science.7545940. [DOI] [PubMed] [Google Scholar]
- Kaplan H. B., Greenberg E. P. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985 Sep;163(3):1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passador L., Cook J. M., Gambello M. J., Rust L., Iglewski B. H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993 May 21;260(5111):1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., Greenberg E. P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper K. R., Beck von Bodman S., Farrand S. K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993 Apr 1;362(6419):448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- Pirhonen M., Flego D., Heikinheimo R., Palva E. T. A small diffusible signal molecule is responsible for the global control of virulence and exoenzyme production in the plant pathogen Erwinia carotovora. EMBO J. 1993 Jun;12(6):2467–2476. doi: 10.1002/j.1460-2075.1993.tb05901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby E. G., Nealson K. H. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica; a model of symbiosis based on bacterial studies. Biol Bull. 1976 Dec;151(3):574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Swartzman A., Kapoor S., Graham A. F., Meighen E. A. A new Vibrio fischeri lux gene precedes a bidirectional termination site for the lux operon. J Bacteriol. 1990 Dec;172(12):6797–6802. doi: 10.1128/jb.172.12.6797-6802.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zhang L., Murphy P. J., Kerr A., Tate M. E. Agrobacterium conjugation and gene regulation by N-acyl-L-homoserine lactones. Nature. 1993 Apr 1;362(6419):446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]