Abstract
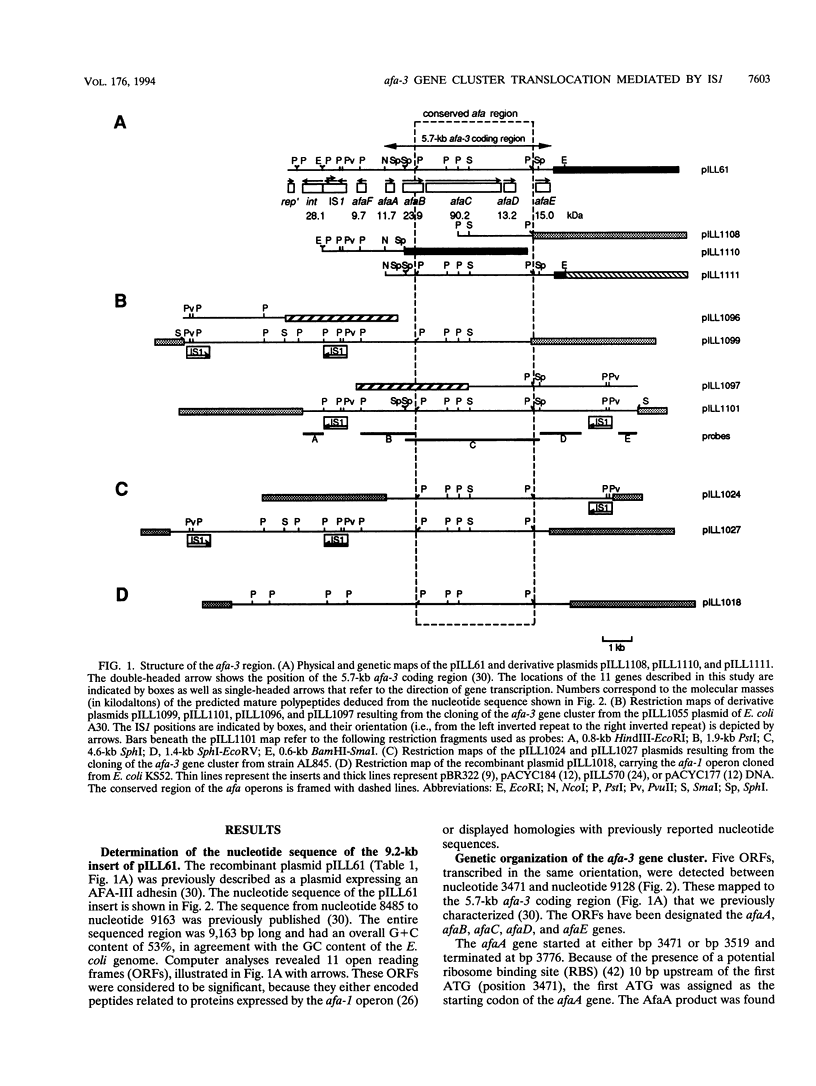
The afa gene clusters encode afimbrial adhesins (AFAs) that are expressed by uropathogenic and diarrhea-associated Escherichia coli strains. The plasmid-borne afa-3 gene cluster is responsible for the biosynthesis of the AFA-III adhesin that belongs to the Dr family of hemagglutinins. Reported in this work is the nucleotide sequence of the 9.2-kb insert of the recombinant plasmid pILL61, which contains the afa-3 gene cluster cloned from a cystitis-associated E. coli strain (A30). The afa-3 gene cluster was shown to contain six open reading frames, designated afaA to afaF. It was organized in two divergent transcriptional units. Five of the six Afa products showed marked homologies with proteins encoded by previously described adhesion systems that allowed us to attribute to each of them a putative function in the biogenesis of the AFA-III adhesin. AfaE was identified as the structural adhesin product, whereas AfaB and AfaC were recognized as periplasmic chaperone and outer membrane anchor proteins, respectively. The AfaA and AfaF products were shown to be homologous to the PapI-PapB transcriptional regulatory proteins. No function could be attributed to the AfaD product, the gene of which was previously shown to be dispensable for the synthesis of a functional adhesin. Upstream of the afa-3 gene cluster, a 1.2-kb region was found to be 96% identical to the RepFIB sequence of one of the enterotoxigenic E. coli plasmids (P307), suggesting a common ancestor plasmid. This region contains an integrase-like gene (int). Sequence analysis revealed the presence of an IS1 element between the int gene and the afa-3 gene cluster. Two other IS1 elements were detected and located in the vicinity of the afa-3 gene cluster by hybridization experiments. The afa-3 gene cluster was therefore found to be flanked by two IS1 elements in direct orientation and two in opposite orientations. The afa-3 gene cluster, flanked by two directly oriented IS1 elements, was shown to translocate from a recombinant plasmid to the E. coli chromosome. This translocation event occurred via IS1-specific recombination mediated by a recA-independent mechanism.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrens R., Ott M., Ritter A., Hoschützky H., Bühler T., Lottspeich F., Boulnois G. J., Jann K., Hacker J. Genetic analysis of the gene cluster encoding nonfimbrial adhesin I from an Escherichia coli uropathogen. Infect Immun. 1993 Jun;61(6):2505–2512. doi: 10.1128/iai.61.6.2505-2512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B. L., Gerlach G. F., Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J Bacteriol. 1991 Jan;173(2):916–920. doi: 10.1128/jb.173.2.916-920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambaud M., Courcoux P., Labigne-Roussel A. Detection by molecular hybridization of pap, afa, and sfa adherence systems in Escherichia coli strains associated with urinary and enteral infections. Ann Inst Pasteur Microbiol. 1988 Sep-Oct;139(5):575–588. doi: 10.1016/0769-2609(88)90156-1. [DOI] [PubMed] [Google Scholar]
- Argos P., Landy A., Abremski K., Egan J. B., Haggard-Ljungquist E., Hoess R. H., Kahn M. L., Kalionis B., Narayana S. V., Pierson L. S., 3rd The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986 Feb;5(2):433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge S. S., Apostol J. M., Jr, Aldape M. A., Moseley S. L. mRNA processing independent of RNase III and RNase E in the expression of the F1845 fimbrial adhesin of Escherichia coli. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1455–1459. doi: 10.1073/pnas.90.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilge S. S., Apostol J. M., Jr, Fullner K. J., Moseley S. L. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993 Mar;7(6):993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- Bilge S. S., Clausen C. R., Lau W., Moseley S. L. Molecular characterization of a fimbrial adhesin, F1845, mediating diffuse adherence of diarrhea-associated Escherichia coli to HEp-2 cells. J Bacteriol. 1989 Aug;171(8):4281–4289. doi: 10.1128/jb.171.8.4281-4289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Braedt G. Different reading frames are responsible for IS1-dependent deletions and recombination. Genetics. 1988 Apr;118(4):561–570. doi: 10.1093/genetics/118.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Båga M., Göransson M., Normark S., Uhlin B. E. Transcriptional activation of a pap pilus virulence operon from uropathogenic Escherichia coli. EMBO J. 1985 Dec 30;4(13B):3887–3893. doi: 10.1002/j.1460-2075.1985.tb04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussac V., Ferrero R. L., Labigne A. Expression of Helicobacter pylori urease genes in Escherichia coli grown under nitrogen-limiting conditions. J Bacteriol. 1992 Apr;174(8):2466–2473. doi: 10.1128/jb.174.8.2466-2473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M. J., Kinsey N. E., Vila J., Kadner R. J. Nucleotide sequence of a 13.9 kb segment of the 90 kb virulence plasmid of Salmonella typhimurium: the presence of fimbrial biosynthetic genes. Mol Microbiol. 1993 May;8(3):543–558. doi: 10.1111/j.1365-2958.1993.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Galyov E. E., Karlishev A. V., Chernovskaya T. V., Dolgikh D. A., Smirnov OYu, Volkovoy K. I., Abramov V. M., Zav'yalov V. P. Expression of the envelope antigen F1 of Yersinia pestis is mediated by the product of caf1M gene having homology with the chaperone protein PapD of Escherichia coli. FEBS Lett. 1991 Jul 29;286(1-2):79–82. doi: 10.1016/0014-5793(91)80945-y. [DOI] [PubMed] [Google Scholar]
- Gay P., Le Coq D., Steinmetz M., Berkelman T., Kado C. I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985 Nov;164(2):918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A., Kuehn M. J., Brändén C. I., Hultgren S. J. Conserved immunoglobulin-like features in a family of periplasmic pilus chaperones in bacteria. EMBO J. 1992 Apr;11(4):1617–1622. doi: 10.1002/j.1460-2075.1992.tb05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Kuehn M., Hultgren S. J. A novel secretion apparatus for the assembly of adhesive bacterial pili. Trends Microbiol. 1993 May;1(2):50–55. doi: 10.1016/0966-842x(93)90032-m. [DOI] [PubMed] [Google Scholar]
- Johnsrud L., Calos M. P., Miller J. H. The transposon Tn9 generates a 9 bp repeated sequence during integration. Cell. 1978 Dec;15(4):1209–1219. doi: 10.1016/0092-8674(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Johnsrud L. DNA sequence of the transposable element IS1. Mol Gen Genet. 1979 Jan 31;169(2):213–218. doi: 10.1007/BF00271673. [DOI] [PubMed] [Google Scholar]
- Jones C. H., Pinkner J. S., Nicholes A. V., Slonim L. N., Abraham S. N., Hultgren S. J. FimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8397–8401. doi: 10.1073/pnas.90.18.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlyshev A. V., Galyov E. E., Smirnov OYu, Guzayev A. P., Abramov V. M., Zav'yalov V. P. A new gene of the f1 operon of Y. pestis involved in the capsule biogenesis. FEBS Lett. 1992 Feb 3;297(1-2):77–80. doi: 10.1016/0014-5793(92)80331-a. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. The fimD gene required for cell surface localization of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1990 Jan;220(2):334–338. doi: 10.1007/BF00260505. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Falkow S. Distribution and degree of heterogeneity of the afimbrial-adhesin-encoding operon (afa) among uropathogenic Escherichia coli isolates. Infect Immun. 1988 Mar;56(3):640–648. doi: 10.1128/iai.56.3.640-648.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne A., Cussac V., Courcoux P. Shuttle cloning and nucleotide sequences of Helicobacter pylori genes responsible for urease activity. J Bacteriol. 1991 Mar;173(6):1920–1931. doi: 10.1128/jb.173.6.1920-1931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D., de Feyter R., Kennedy M., Phua S. H., Semon D. D protein of miniF plasmid acts as a repressor of transcription and as a site-specific resolvase. Nucleic Acids Res. 1986 Dec 22;14(24):9713–9728. [PMC free article] [PubMed] [Google Scholar]
- Le Bouguenec C., Archambaud M., Labigne A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J Clin Microbiol. 1992 May;30(5):1189–1193. doi: 10.1128/jcm.30.5.1189-1193.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bouguenec C., Garcia M. I., Ouin V., Desperrier J. M., Gounon P., Labigne A. Characterization of plasmid-borne afa-3 gene clusters encoding afimbrial adhesins expressed by Escherichia coli strains associated with intestinal or urinary tract infections. Infect Immun. 1993 Dec;61(12):5106–5114. doi: 10.1128/iai.61.12.5106-5114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Båga M., Tennent J. M., Normark S. Nucleotide sequence, regulation and functional analysis of the papC gene required for cell surface localization of Pap pili of uropathogenic Escherichia coli. Mol Microbiol. 1987 Sep;1(2):169–178. doi: 10.1111/j.1365-2958.1987.tb00509.x. [DOI] [PubMed] [Google Scholar]
- Nou X., Skinner B., Braaten B., Blyn L., Hirsch D., Low D. Regulation of pyelonephritis-associated pili phase-variation in Escherichia coli: binding of the PapI and the Lrp regulatory proteins is controlled by DNA methylation. Mol Microbiol. 1993 Feb;7(4):545–553. doi: 10.1111/j.1365-2958.1993.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Nowicki B., Svanborg-Edén C., Hull R., Hull S. Molecular analysis and epidemiology of the Dr hemagglutinin of uropathogenic Escherichia coli. Infect Immun. 1989 Feb;57(2):446–451. doi: 10.1128/iai.57.2.446-451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Saul D., Spiers A. J., McAnulty J., Gibbs M. G., Bergquist P. L., Hill D. F. Nucleotide sequence and replication characteristics of RepFIB, a basic replicon of IncF plasmids. J Bacteriol. 1989 May;171(5):2697–2707. doi: 10.1128/jb.171.5.2697-2707.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spiers A. J., Bhana N., Bergquist P. L. Regulatory interactions between RepA, an essential replication protein, and the DNA repeats of RepFIB from plasmid P307. J Bacteriol. 1993 Jul;175(13):4016–4024. doi: 10.1128/jb.175.13.4016-4024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W., Schmidt M. A., Labigne-Roussel A. F., Falkow S., Schoolnik G. AFA-I, a cloned afimbrial X-type adhesin from a human pyelonephritic Escherichia coli strain. Purification and chemical, functional and serologic characterization. Eur J Biochem. 1985 Oct 15;152(2):315–321. doi: 10.1111/j.1432-1033.1985.tb09200.x. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]