Abstract
During embryonic development of brain laminated structures, the protein Reelin, secreted into the extracellular matrix of the cortex and hippocampus by Cajal–Retzius (CR) cells located in the marginal zone, contributes to the regulation of migration and positioning of cortical and hippocampal neurons that do not synthesize Reelin. Soon after birth, the CR cells decrease, and they virtually disappear during the following 3 weeks. Despite their disappearance, we can quantify Reelin mRNA (approximately 200 amol/μg of total RNA) and visualize it by in situ hybridization, and we detect the translated product of this mRNA by immunocytochemistry preferentially in γ-aminobutyric acid (GABA)ergic neurons of adult rat cortex and hippocampus. In adult rat cerebellum, Reelin is expressed in glutamatergic neurons (granule cells). The translated product of this mRNA is readily exported from the granule cell somata to the parallel fibers, where it has been detected by electron microscopy in axon terminals located presynaptically to Purkinje cell dendrites.
The neurological mouse phenotype dubbed “reeler” derives its appellation from a characteristic reeling gait caused by impaired regulation of motor coordination and ataxia (for a review, see ref. 1). In 1995, a gene was cloned and the structure of the protein encoded by this gene was revealed; this protein, termed Reelin (2), was identified as the product of a gene that, when mutated, is responsible for the onset of the neurological phenotype expressed by the reeler mouse (2–4). Probably the reeler mouse brain becomes scrambled because of a mutation-dependent dysfunction of Reelin. Consequently, many neurons cannot reach their proper final destination, and it is also probable that their axons and neurites cannot branch appropriately (3, 5–7). During embryogenesis, Reelin is released near the final destination of the migrating cortical neurons, perhaps acting as a signpost for these migrating neurons (3, 6–10). The identification of Reelin and the recent progress in Reelin research was brought about by the successful preparation of a monoclonal “allogenic” antibody termed CR50, which recognizes a brain protein expressed in normal mice but not in the reeler mouse brain (10). The epitope recognized by the CR50 antibody appears to be located at the NH2 terminus of Reelin (10). We know five reeler mouse alleles: the original reeler (Relnrrl) (11); the Orleans allele Relnor-rl (4); the transgenic allele Relnrl-tg (2); and Relnrl-Ab1 and Relnrl-Alb2, two alleles generated by chlorambucil mitogenesis (12, 13). The intrinsic molecular mechanisms that are operative in evoking the neurological reeler phenotype may differentially affect Reelin function in each of the five different phenotypes. The complete gene deletions per se may be a factor operative in the expression of the neurological phenotype Relnrl-tg (2), but in other phenotypes, Reelin gene deletions, though modest in extension, prevent Reelin transcription or secretion (4, 12, 14). In the cortex and hippocampus of rat embryos, Reelin begins to be synthesized in Cajal–Retzius (CR) cells from embryonic day 13 to the second postnatal week, and in the cerebellum Reelin is expressed first in the external granule cell layer (EGL) before the granule cell migration to the internal granule cell layer (IGL) (2, 15–17). When the CR50 antibody is added to embryonic preparations expressing normal histogenetic patterns of lamination, it induces typical histogenetic abnormalities of the Relnrl phenotype (7, 9, 10, 17). After birth, with the disappearance of the pioneer cells, the expression of Reelin mRNA and that of the translated product is reduced but not abolished (2, 8, 15, 18, 19). However, it cannot be categorically excluded that some pioneer neurons remain in the brain, migrate to deeper cortical layers (20), and become responsible for the postnatal production of Reelin.
The present paper is concerned with experiments directed to establish the quantitative expression of Reelin mRNA in the adult rat brain with reverse transcriptase (RT)-PCR and to document the exact cell location of Reelin production in various brain structures of adult rats with in situ hybridization and immunocytochemistry, using confocal or electron microscopy. This work shows that in the cortex and hippocampus of the adult rat, Reelin is expressed almost exclusively in γ-aminobutyric acid-(GABA)ergic interneurons; whereas in the cerebellum, Reelin is expressed almost exclusively in glutamatergic granule cells. Thus in cerebellum, in which the output is coded by GABAergic neurons, Reelin is located in glutamatergic neurons; and in the hippocampus, where the functional output is coded by glutamatergic mechanisms, Reelin preferentially is expressed and secreted by GABAergic interneurons. The latest breakthrough in the Reelin story is the identification (21, 22) of a scrambler mutation (23), which produces a reeler-like phenotype, but this scrambler mutation maps on chromosome 4, whereas the reeler mutation maps on chromosome 5. The scrambler locus was considered a Reelin receptor candidate; however, it was found that one of the two genes that are homologous to the Drosophila “disabled”-like scrambler maps on chromosome 4 (24). This gene is the mouse disabled homologue 1 (mDab1) and functions as an essential component of Reelin response; it is expressed intracellularly in Reelin target neurons but is not an extracellularly located Reelin receptor. To establish Reelin function in the adult rat brain, one may first have to determine whether Reelin and mDabl protein (21, 22) work in partnership as they likely do in embryogenesis, and whether Reelin regulates the direction of axon sprouting and that of dendritic arborization, thereby protecting the function of various neuronal circuits from an invasion by sprouting axons and dendrites (25).
MATERIALS AND METHODS
Competitive, Quantitative RT-PCR Analysis.
Quantitative RT-PCR was performed as described by Impagnatiello et al. (26).
To determine whether full-length Reelin mRNA is expressed in adult rats, we used two sets of PCR primers: set I, forward primers base pairs 1264–1285 and reverse primers base pairs 1555–1576; and set II, forward primers base pairs 9211–9231 and reverse primers base pairs 9549–9569 (mouse cDNA sequence, GenBank accession no. U24703). Reelin internal standard templates extending from base pair 1264 to base pair 1576 (internal standard 1°) and from base pair 9211 to base pair 9669 (internal standard 2°) were generated by site-directed mutagenesis (24) using PCR overlapping extension to introduce a BanI restriction endonuclease site for the internal standard 1° and BglII restriction endonuclease site for the internal standard 2° midway between the amplification primers. The constructed internal standard cDNAs were subcloned into the HincII site of the pGEM1 vector by using standard cloning methodology; the base sequence, the location of the mutation, and the direction of the insert were verified. To obtain internal standard cRNA, the vector was linearized 914 bp downstream of the insert by using SspI restriction endonuclease and finally transcribed with T7 RNA polymerase I to obtain the sense strand cRNA (27).
Immunocytochemistry.
Rats were anesthetized and perfused transcardially with 100 ml of saline followed by 100 ml of ice-cold 4% paraformaldehyde in phosphate-buffered saline (PBS: 137 mM NaCl/2.7 mM KCl/5 mM Na2HPO4/1.7 mM KH2PO4, pH 7.4) and placed in fixative for 24 hr. Brains were then embedded in 30% sucrose, frozen, and sectioned in a cryostat at 20 μm. After a thorough washing in PBS, free-floating sections were blocked with 3% normal goat serum incubated overnight at 4°C with the G-10 antibody (13) [1:1000 dilution in 1% normal goat serum in Tris-buffered saline (TBS), pH 7.4, containing 0.25% Triton X-100 (TBST)], which specifically recognizes an epitope close to the NH2 terminus of the Reelin protein. The following day, the samples were washed several times in 1% normal goat serum in TBS before being incubated for 1 hr with a biotinylated secondary antibody. After washing, sections were processed with the avidin biotinylated complex (ABC; Vector Laboratories) and the reaction was developed with 3,3′-diaminobenzidine (DAB). Sections were mounted on slides and counterstained with neutral red.
Some sections of adult rats were processed for double-labeling to detect the presence of Reelin mRNA (in situ hybridization) and the GABA-synthesizing enzyme glutamic acid decarboxylase67 GAD67 (immunocytochemistry). Sections from perfused brains (see above protocol) were thoroughly washed at 4°C with diethyl pyrocarbonate (DEPC)/PBS containing 0.02% Tween 20. After a second wash with DEPC/PBS, floating sections were incubated overnight at 42°C with a hybridization cocktail, including an antisense oligonucleotide probe (oligoprobe) for Reelin (1264–1311 bp) biotinylated at positions 1267, 1278, 1289, 1299, and 1307 (DNA International, Lake Oswego, OR). Reelin sense (base pairs 1264–1311) and antisense (base pairs 10225–10282) oligoprobes were used for in situ hybridization. The following day, the samples were washed at high stringency with SSC (150 mM NaCl/15 mM sodium citrate, pH 7) and PBS followed by 30 min in 1% BSA in PBS. The sections were incubated overnight at 4°C with both mouse anti-biotin (primary antibody in situ) and rabbit anti-GAD67 (primary antibody, immunocytochemistry) antibodies. The sections were washed in 1% BSA in PBS at 4°C, incubated 1 hr at room temperature with biotinylated anti-mouse antibody (secondary antibody, in situ), washed, and then incubated for 1 hr at room temperature with a fluorescein-conjugated antibody against biotin (tertiary antibody, in situ) and a rhodamine-conjugated anti-rabbit immunoglobulin antibody (secondary antibody immunocytochemistry). Mounted sections were studied with a Leica TCS-NT laser confocal microscope. To quantify the number of neurons coexpressing Reelin mRNA and GAD67, brain coronal sections (three consecutive sections from each animal) were studied. From each section, six microscopic fields (three for each hemisphere) were analyzed. For each brain, we first identified Reelin mRNA-positive neurons (fluorescein fluorescence) and then established the percentage of these neurons that contained GAD67-rhodamine fluorescence. In the same slide, we first identified GAD67 (rhodamine fluorescence)-positive neurons, and then established the percentage of neurons that were Reelin mRNA positive. For each cortical layer in each section, we counted approximately 100 Reelin- or GAD-positive cells. For CA1, CA2, and CA3 hippocampal regions and for the dentate gyrus, where the Reelin- and GAD67-positive neurons were less abundant, all the Reelin mRNA- and GAD-positive cells of the microscopic fields were counted.
Electron Microscopy.
Nine-day-old and adult rats were anesthetized and perfused transcardially with PBS followed by a fixative (4% paraformaldehyde and 0.1% glutaraldehyde in PBS). Brains were removed and left overnight in fixative, and 80-μm sections were taken through the cerebellum by using a Vibratome. Sections were preincubated in RPMI medium 1640 (GIBCO) containing 2% normal goat serum and 1% BSA in PBS, each for 30 min, before being incubated overnight with the G-10 monoclonal antibody diluted (1:1000) in 1% BSA. Subsequently, sections were washed and incubated with antibodies tagged with gold particles 1 nm in size. After frequent washes in 1% BSA, the size of the gold particles was increased by incubating for 7 min in a silver-enhancement solution (Goldmark Biologicals, Phillipsburg, NJ). This reaction was stopped by washing the sample with double-distilled water. Small blocks of cerebellar cortex were then osmicated, dehydrated, and embedded in an Epon-like resin. Thin sections were contrasted with uranyl acetate and lead citrate, observed, and then photographed, using a Zeiss-902 electron microscope.
RESULTS
Reelin Expression in the Brain Structures of Neonatal Rats.
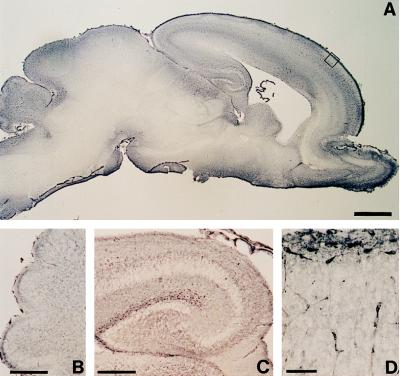
After birth, Reelin-immunoreactive neurons are found in the marginal zone of the cerebral cortex (Fig. 1A), in the subiculum and hippocampal fissure (Fig. 1A), in the olfactory bulb (Fig. 1A), and in the inner part of the EGL of the cerebellum (Fig. 1B). In the marginal zone of the cortex, the immunolabeling of Reelin is evident not only in the cytoplasm of CR cells but also in the extracellular matrix surrounding these neurons, where it is greater than in the deeper cortical layers (Fig. 1D).
Figure 1.
Reelin (G10)-immunostaining in the neonatal [postnatal day 0 (P0)] rat. Low magnification of a brain sagittal section (A) shows that Reelin is most abundant in the marginal zone of the cerebral cortex, hippocampus, cerebellum, and olfactory bulb. Higher magnification of the cerebellum (B) shows that Reelin is most abundant in the EGL. C shows that the highest Reelin expression is around the hippocampal fissure. D, which is a high magnification of the cortical plate boxed in A, shows that Reelin is highly expressed in large horizontally oriented fusiform neurons, presumably CR cells. [Bars = 1 mm (A), 25 μm (B and C), and 50 μm (D).]
Combined in situ hybridization and immunohistochemistry of brain tissue at birth shows that Reelin mRNA in the cortex and the hippocampus is mostly present in CR cells identified as GABAergic (for an example, see Fig. 2). Electron micrographs of the cerebellar granule neurons of 9-day-old rats show that neurons located in the outer part of the EGL are immunonegative (Fig. 3A), whereas granule cells in the inner part of the EGL (Fig. 3B) as well as granule cells in the internal granule layer (IGL) (Fig. 3C) are actively producing Reelin as evidenced by the abundant clusters of gold particles in the cytoplasm of these cells. Labeling is also seen in the extracellular matrix surrounding these neurons (Fig. 3C).
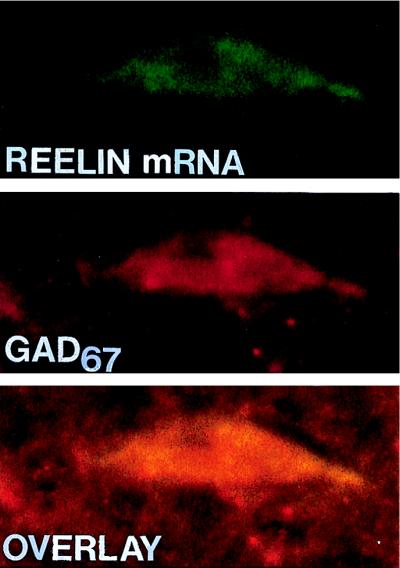
Figure 2.
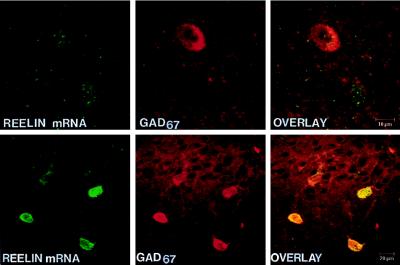
Confocal laser microscope images of Reelin mRNA detected by in situ hybridization using a biotinylated oligoprobe (base pairs 1264–1311) visualized with fluorescein (green) (Top), and GAD67 immunohistochemistry visualized with rhodamine (red) (Middle), in a hippocampal CR cell at P0. (Bottom) Overlay of Top and Middle showing that Reelin mRNA colocalizes with GAD67 (yellow). (×4,000.)
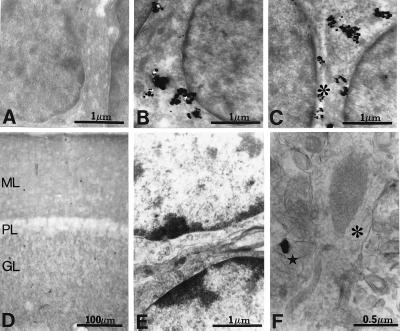
Figure 3.
Electron micrographs of Reelin immunolabeling in rat cerebellum. (A–C) Electron micrographs of Reelin gold immunolabeling in P9 rat cerebellum. (A) Absence of gold immunolabeling in the outer part of the granule cell layer. (B) Abundance of gold immunolabeling in neuronal somata of EGL (inner part). (C) Gold immunolabeling in neuronal somata of internal granule cell layer (asterisks indicates “secreted” Reelin). (D) Light micrograph of adult rat cerebellum DAB immunostained for Reelin. Note that Reelin is abundant in the molecular (ML) and granule cell layer (GL) but absent from the Purkinje cell layer (PL). (E) Electron micrograph of the granule cell layer, showing absence of Reelin immunolabeling. (F) Electron micrograph of the molecular layer. Note that the Purkinje cell dendrite crossing the picture from bottom to top includes a large mitochondrion (asterisk). One of the neighboring parallel fibers shows Reelin gold immunolabeling (star).
Reelin Expression in the Telencephalon of Adult Rats.
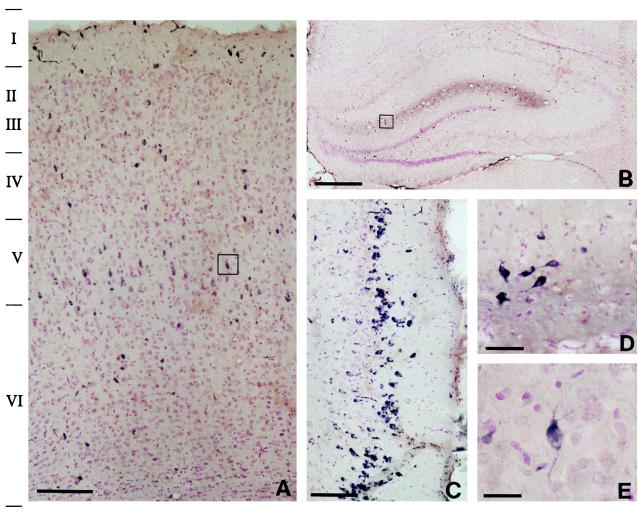
In the cingulate, motor, and somatosensory cortex, Reelin immunoreactivity is expressed in scattered neurons detectable in every cortical layer. Fig. 4 shows that the greatest number of Reelin-positive cells in the parietal cortex is found in layers I/III and V/VI (Fig. 4A). In Reelin-containing neurons, immunolabeling is evident in both somata and proximal dendrites (Fig. 4E). The RT-PCR assay using forward primers (base pairs 1264–1285) and reverse primers (base pairs 1555–1576) that target the region close to the 5′ end of the Reelin cDNA shows that the cortex of the adult rat contains 220 ± 18 (±SEM) attomoles (amol) of Reelin/μg of total RNA (n = 6). Similarly, quantitative RT-PCR analysis using forward primers (base pairs 9211–9231) and reverse primers (base pairs 9549–9569) that target the 3′ end of the Reelin cDNA yielded almost identical results (210 ± 22 amol/μg of total RNA). In situ hybridization of Reelin mRNA (antisense strand oligoprobe base pairs 1264–1311) combined with GAD67 immunohistochemistry indicate that the majority of the above-mentioned neurons expressing Reelin are GABAergic (see Fig. 5 and Table 1). Approximately half the cortical GABAergic interneurons express Reelin (see Table 1). In situ hybridization experiments show similar results when Reelin antisense strand oligoprobe (base pairs 10225–10282) is used.
Figure 4.
Light micrograph of adult rat brain DAB immunostained for Reelin (blue) and counterstained with neutral red for cells (red). A illustrates that Reelin-positive cells are found throughout the cortical laminae but are particularly numerous in layer V. B shows a diffuse Reelin band lining the hippocampal fissure, in addition to numerous Reelin-positive cells around this fissure, in the stratum oriens, and in the hilus of the dentate gyrus. C shows a high density of Reelin-positive cells in entorhinal cortex layer II. D is a high magnification of the boxed area of B showing Reelin-positive cells and extracellular reelin around the hippocampal fissure. E is a high magnification of the box in A illustrating Reelin expressed in somata and proximal dendrites of a cortical bitufted cell. [Bars = 150 μm (A), 500 μm (B), 125 μm (C), 50 μm (D), and 25 μm (E).]
Figure 5.
Confocal microscope images of Reelin mRNA detected by in situ hybridization using a biotinylated oligoprobe (base pairs 1264–1311) visualized with fluorescein (green) and GAD67 immunolabeling visualized with rhodamine (red) in adult rat neocortex (Upper) and hippocampus (Lower). The overlay of the Reelin and GAD67 distribution shows that in the cortex mRNA for Reelin is found in both GABAergic and non-GABAergic cells, whereas in the hippocampus (CA1) all Reelin-positive cells are GABAergic. Similar results were obtained with Reelin antisense strand oligoprobe (base pairs 10225–10282).
Table 1.
Neuronal colocalization of GAD67 and reelin
| Location | Reelin-positive neurons expressing GAD67, % | GAD67-positive neurons expressing Reelin, % |
|---|---|---|
| Frontal cortex | 70 ± 6.7 | |
| Layers I–III | 51 ± 4.1 | |
| Layers IV and V | 60 ± 4.7 | |
| Layer VI | 65 ± 5.2 | |
| Hippocampus | ||
| CA1 | 95 ± 3.5 | |
| Stratum oriens | 88 ± 6.5 | |
| Stratum radiatum | 45 ± 4.2 | |
| St. lacunosum-moleculare | 40 ± 4.0 | |
| CA2/CA3 | 93 ± 6.3 | |
| Stratum oriens | 85 ± 6.7 | |
| Stratum radiatum | 55 ± 5.2 | |
| St. lacunosum-moleculare | 50 ± 4.6 | |
| Dentate gyrus | 97 ± 3.6 | |
| Basket neurons | 85 ± 6.4 | |
| Polymorphic layer | 70 ± 5.2 | |
| Molecular layer | 24 ± 4.7 |
Each value is the mean ± SEM of five animals. Control sections hybridized with sense probes for Reelin or omitting GAD67 primary antibody failed to produce fluorescence.
In the CA1, CA2, and CA3 regions of the hippocampus, there is a high expression of Reelin-positive cells in stratum oriens, and a more moderate expression in the stratum radiatum and stratum lacunosum-moleculare (Fig. 4B). In the dentate gyrus, Reelin is expressed predominantly in the interneurons located at the base of the granule cell layer (but not in the granule cells) and in the polymorphic layer, whereas in the molecular layer Reelin-containing neurons are sparse (Fig. 4B). In the areas surrounding the hippocampal fissure (stratum lacunosum-moleculare, and the upper third of the molecular layer of the dentate gyrus) a strong diffuse Reelin immunolabeling is detected (Fig. 4 B and D). Double labeling for Reelin mRNA and GAD67 (see Fig. 5) shows that every hippocampal neuron that expresses Reelin is GABAergic, including the great majority of GABAergic cells in the stratum oriens of areas CA1, CA2, and CA3 (Table 1). However, the percentage of GABAergic cells that express Reelin mRNA in other hippocampal layers is low (Table 1). In the dentate gyrus, Reelin-positive neurons include pyramidal GABAergic basket cells in the granule layer, as well as most GABAergic interneurons of the polymorphic layer, whereas only about 25% of the GABAergic cells of the molecular layer express Reelin (Table 1). In the entorhinal cortex most of the Reelin immunoreactive neurons are located in layer II (Fig. 4C). We have not yet established what proportion of these neurons express GAD67.
Reelin Expression in Adult Rat Cerebellum.
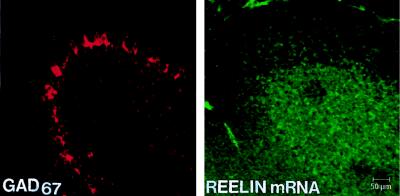
In adult rat cerebella, the RT-PCR technique indicates that Reelin mRNA expression is 495 ± 18 amol/μg of total RNA; this content is approximately 2.5-fold higher than that of the cortex. In situ hybridization demonstrates that Reelin mRNA is expressed mostly in the granular cell layer, but is virtually absent in the molecular layer or in the Purkinje cells, which are GAD67-positive (Fig. 6). In contrast, immunolabeling at the optical microscope level reveals that although in the granular layer there is sparsely distributed immunostaining, a stronger signal is seen in the molecular layer, where Reelin labeling is diffuse and appears to be located in the neuropil (Fig. 3D). Electron microscopy reveals that many cells in the granular layer fail to show Reelin immunoreactivity (Fig. 3E), whereas there are scattered gold particles in the molecular layer associated with parallel fibers proximal to their innervation of Purkinje cell dendrites (Fig. 3F).
Figure 6.
Confocal microscope images of adult rat cerebellum, showing the distribution of GAD67 (immunolabeled with rhodamine: red) and Reelin mRNA (detected with biotinylated oligoprobe base pairs 1264–1311 and immunolabeled with fluorescein: green). Note that GAD67 is found predominantly in the Purkinje cell layer (Left), whereas mRNA for Reelin is found primarily in the granule cell layer (Right).
DISCUSSION
This study demonstrates that Reelin, which in the developing brain is generally produced by CR cells in the marginal zone of the cortex and hippocampus and by the cerebellar granule cells in the EGL, is also expressed in significant amounts in the adult rat brain, where it is expressed in permanent neurons located deeper in the lamination of the cortex, hippocampus, and cerebellum. We show that in the hippocampal formation, Reelin mRNA and its cognate protein are expressed almost exclusively in GAD67-expressing neurons. In the cortex, scattered Reelin mRNA-positive or Reelin immunopositive neurons are present in all layers, but are most abundant in layers I/III, and V/VI; a high proportion of these neurons are GABAergic, as revealed by immunopositive staining with GAD67 antibodies. In contrast, in the cerebellum Reelin mRNA is preferentially expressed in the glutamatergic granule cell layer, whereas the protein immunostaining is abundant mostly in the molecular layer.
Table 1 summarizes our findings: In the cortex of the adult rat, the number of Reelin-positive cells that are GABAergic is about 70%. In contrast, the percentage of cells that are GABAergic and express Reelin varies between 51% and 65% in different cortical layers. In the hippocampus, Reelin-containing cells that are GABAergic range between 93% and 95%, whereas the cells that are GABAergic and express Reelin are 85–88% in the stratum oriens, 45–55% in the stratum radiatum, and 40–50% in the stratum lacunosum-moleculare. In the dentate gyrus, 97% of the Reelin-expressing cells are GABAergic and represent 85% of the basket cells, 70% of the GABAergic cells in the polymorphic layer, and 24% of those in the molecular layer. In contrast, in the cerebellum Reelin is expressed in glutamatergic granule cells and not in GABAergic Purkinje cells.
In cortex and hippocampus of adult rats, neurons express a high-intensity Reelin mRNA signal, which is comparable to that detected at birth in CR cells. Thus, even though Reelin-positive neurons in the adult rat cortex are scattered in different layers, the high mRNA level in each cell justifies the significant amount of Reelin mRNA measured by RT-PCR in homogenates prepared from cortices of adult rats. In contrast, in the cerebellar granule cells of adult rats, the density of the Reelin mRNA signal is weaker than that found in the cerebellar granule neurons of rats studied immediately after birth. However, the high number of granule cells expressed in the cerebellum may explain why the mRNA level measured by RT-PCR is higher in cerebellar than in cortical homogenates.
Because the concentration of Reelin in brain extracts is not sufficient to characterize the protein by using the Western blotting technique, it is difficult to conclude from our immunohistochemical studies whether the Reelin expressed in the adult brain is different from the full-length protein expressed in the embryonic brain (3, 13). Indirectly, we have attempted to answer this questions by using: (i) quantitative RT-PCR with internal standards that recognize Reelin oligonucleotide regions near the 5′ or near the 3′ end of the cDNA; and (i) by in situ histochemistry using antisense oligoprobes designed for the 5′ or the 3′ end of Reelin cDNA. Using this approach, we have demonstrated that (i) the amounts of Reelin mRNA in the cortex and cerebellum are identical when measured with two internal standards that span virtually the entire length of the Reelin cDNA; (ii) the distribution and density of the in situ hybridization signal for Reelin mRNA revealed an identical brain pattern with either the 5′ or the 3′ end targeted oligoprobes; (iii) immunochemical studies with G-10 antibodies, which recognize Reelin’s NH2-terminal region (13), also reveal a pattern of distribution quantitatively similar to that of in situ hybridization studies. These data support the view, but do not definitively prove, that full-length Reelin is expressed in various structures of adult rat brain.
An important finding from our Reelin immunolabeling studies is that in the cortex, hippocampus, and cerebellum of adult rat brain, similar to the developing brain (3, 10, 18), the Reelin immunoreactivity not only is expressed in neurons but also is found extraneuronally. For example, the extracellular Reelin staining pattern revealed immunohistochemically deep in the lacunosum-moleculare layer of the hippocampus at the border of the rudimentary hippocampal fissure allows the inference that Reelin may be released from terminals of local GABAergic interneurons, or alternatively, may be released from afferent axon terminals of the perforant pathway that originates from Reelin-expressing layer II neurons of the entorhinal cortex (Fig. 4C). As supporting evidence for a Reelin transport from somata to axon terminal and for its secretion into the extracellular matrix in the brain of adult rats, we present the results of our electron microscopic studies conducted on subcellular Reelin localization (Fig. 3 E and F). In the adult cerebellum, weakly stained cerebellar granule cell bodies (Fig. 3 D and E) give rise to Reelin immunostaining in parallel fiber nerve terminals (Fig. 3 D and E) and to Reelin in the extracellular matrix space (Fig. 3F), as suggested also by the diffuse immunostaining present in the molecular cell layer (Fig. 3D). Notably, Reelin mRNA expression is confined to the granule cell layer, with virtually no signal for Reelin mRNA expression in the molecular layer (Fig. 6).
These findings invite speculation that Reelin produced by the granule cells of the adult rat cerebellum is transported to the axon terminals of the parallel fibers (Fig. 3F) and then secreted extracellularly. Probably it acts at a short distance in a paracrine fashion at specific sites on extracellular matrix proteins or Purkinje cell dendrites, or on autoreceptors of the granule cell axon terminals.
The characteristic cell distribution of Reelin mRNA and its translation product, and the presence of diffuse Reelin immunostaining in the neuropil of the cortex and hippocampus of adult rats, predict that, as in the granule cells of the cerebellum, Reelin may be secreted from the nerve terminals of neurons that colocalize Reelin mRNA and GAD67 and act in a paracrine fashion on pyramidal cells of layers II to VI of the cortex, or of the CA1, CA2, and CA3 regions of the hippocampus. However, this suggestion could lead to a simplistic interpretation of the microanatomy of cortical and hippocampal Reelin. Our histochemical studies suggest that a number of questions should be addressed. In the frontal cortex, 30% of Reelin-secreting cells are not GABAergic. What transmitter coexists with Reelin in these non-GABAergic neurons? Will these cells be glutamatergic? Will they secrete the Reelin they synthesize in a manner similar to that of GABAergic neurons? Do these cells have another specific role? Do they synapse on GABAergic neurons that contain Reelin or in GABAergic neurons that do not contain Reelin? Can we extrapolate concepts on Reelin biosynthesis and release regulation acquired from the studies of the developing brain to Reelin biosynthesis and secretion in the adult rat brain? We believe that some of these questions may be answered by electrophysiological experiments on mice with an inducible knockout Reelin gene that we have initiated.
An important breakthrough in determining the mechanism(s) by which Reelin secreted from the pioneer neurons may exert its effects on target neurons during development is reported by Sheldon et al. (21) and Howell et al. (22). The authors clearly identify the mouse disabled 1 (mDab1) gene as a critical component of a signaling cascade operative in the Reelin response of target neuronal cells. Although mDab1 is not the Reelin receptor, it may participate in the signal transduction triggered by Reelin in the target neurons during development. We plan to test whether the mDab1 gene is expressed in the adult brain as well as verify its cell location in young and adult mice. We also plan to produce an inducible knockout (KO) of the mDab1 gene and to study the consequences of this KO morphologically and electrophysiologically. This will allow conclusions regarding whether Reelin function depends on the mDab1 gene in adult mice. The role of these and other important differences between embryonic and adult Reelin and mDab1 needs to be evaluated before proposing that the Reelin secreted in the developing brain differs from that of the adult brain. Reelin may contribute in many ways to the formation of neuronal circuits in the adult brain by the use of mechanisms similar to those of embryonic development. Reelin might protect circuits from interference due to the sprouting of neurons or dendrites by stopping their elongation in a given direction, as is suggested (25) to occur during embryonic development. Another possibility being investigated is whether in the adult brain Reelin regulates synaptic plasticity, including long-term desensitization (LTD) and long-term potentiation (LTP). In fact, Reelin frequently is released by terminals facing the dendritic spines of Purkinje or pyramidal cells. The experiments with transgenic mice discussed above will be used to study such aspects of Reelin function.
Acknowledgments
We thank Dr. Edward Jones, Department of Anatomy and Neurobiology, University of California Irvine, and Dr. Pasko Rakic, Department of Neurobiology, Yale University, New Haven, CT, for their constructive criticisms and suggestions in the preparation of the manuscript. We also thank Dr. Andre M. Goffinet, Department of Human Physiology, Facultés Universitaires Notre-Dame de la Paix School of Medicine, Namur, Belgium, for the generous gift of anti-Reelin G-10 antiserum.
ABBREVIATIONS
- CR
Cajal–Retzius
- EGL
external granule cell layer
- RT
reverse transcriptase
- GABA
γ-aminobutyric acid
- DAB
3,3′-diaminobenzidine
- GAD67
glutamic acid decarboxylase67
- Pn
postnatal day n
References
- 1.Rakic P, Caviness V S., Jr Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 2.D’Arcangelo G, Miao G G, Chen S C, Soares H D, Morgan J L, Curran T. Nature (London) 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 3.D’Arcangelo G, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Curran T. J Neurosci. 1997;17:23–31. doi: 10.1523/JNEUROSCI.17-01-00023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirotsune S, Takahara T, Sasaki N, Hirose K, Yoshiki A, Ohashi T, Kusakabe M, Murakami Y, Muramatsu M, Watanabe S, Nakao K, Katsuki M, Hayashizaki Y. Nat Genet. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- 5.Caviness V S, Rakic P. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- 6.Del Rio J A, Heimrich B, Super H, Borrell V, Frotscher M, Soriano E. J Neurosci. 1996;16:6896–6907. doi: 10.1523/JNEUROSCI.16-21-06896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Rio J A, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E. Nature (London) 1997;385:70–74. doi: 10.1038/385070a0. [DOI] [PubMed] [Google Scholar]
- 8.DeSilva U, D’Arcangelo G, Braden V V, Chen J, Miao G G, Curran T, Green E D. Genome Res. 1997;7:157–164. doi: 10.1101/gr.7.2.157. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima K, Mikoshiba K, Miyata T, Kudo C, Ogawa M. Proc Natl Acad Sci USA. 1997;94:8196–8201. doi: 10.1073/pnas.94.15.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa M, Miyata T, Nakajima K, Yagyu K, Seike M, Ikenaka K, Yamamoto H, Mikoshiba K. Neuron. 1995;14:899–912. doi: 10.1016/0896-6273(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 11.Falconer D S. J Genet. 1951;50:192–201. doi: 10.1007/BF02996215. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery J C, Guarneri M H, Tartaglia K E, Flaherty L A. Mamm Genome. 1994;5:756–761. doi: 10.1007/BF00292008. [DOI] [PubMed] [Google Scholar]
- 13.DeBergeyck V, Nakajima K, Rouvroit C L, Naerhuyzen B, Goffinet A M, Miyata T, Ogawa M, Mikoshiba K. Brain Res Mol Brain Res. 1997;50:85–90. doi: 10.1016/s0169-328x(97)00166-6. [DOI] [PubMed] [Google Scholar]
- 14.Bar I, Lambert De Rouvroit C, Krizman D B, Demoncourt C, Ruelle D, Beckers M C, Goffinet A M. Genomics. 1995;26:543–549. doi: 10.1016/0888-7543(95)80173-j. [DOI] [PubMed] [Google Scholar]
- 15.Schiffinann S N, Bernier B, Goffinet A M. Eur J Neurosci. 1997;9:1055–1071. doi: 10.1111/j.1460-9568.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyata T, Nakajima K, Aruga J, Takahashi S, Ikenaka K, Mikoshiba K, Ogawa M. J Comp Neurol. 1996;372:215–228. doi: 10.1002/(SICI)1096-9861(19960819)372:2<215::AID-CNE5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Miyata T, Nakajima K, Mikoshiba K, Ogawa M. J Neurosci. 1997;17:3599–3609. doi: 10.1523/JNEUROSCI.17-10-03599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drakew A, Frotscher M, Deller T, Ogawa M, Heimrich B. Neuroscience. 1998;82:10791–10798. doi: 10.1016/s0306-4522(97)00326-6. [DOI] [PubMed] [Google Scholar]
- 19.Caruncho H J, Pesold C, Impagnatiello F, Pisu M G, Miyata T, Ogawa M, Costa E, Guidotti A. Soc Neurosci Abstr. 1997;28:14. [Google Scholar]
- 20.Parnavelas J G, Edmunds S M. J Neurocytol. 1983;12:863–871. doi: 10.1007/BF01258156. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon M, Rice D S, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell B W, Cooper J A, Goldowitz D, Curran T. Nature (London) 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 22.Howell B W, Hawkes R, Soriano P, Cooper J A. Nature (London) 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 23.Sweet H O, Bronson R T, Johnson K R, Cook S A, Davisson M T. Mamm Genome. 1996;7:798–802. doi: 10.1007/s003359900240. [DOI] [PubMed] [Google Scholar]
- 24.Howell B W, Gertler F B, Cooper J A. EMBO J. 1997;16:121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frotscher M. Cell Tissue Res. 1997;290:315–322. doi: 10.1007/s004410050936. [DOI] [PubMed] [Google Scholar]
- 26.Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy J M, Costa E, Guidotti A. Mol Pharmacol. 1996;49:822–831. [PubMed] [Google Scholar]
- 27.Grayson D R, Bovolin P, Santi M R. Methods Neurosci. 1993;12:191–208. [Google Scholar]