Abstract
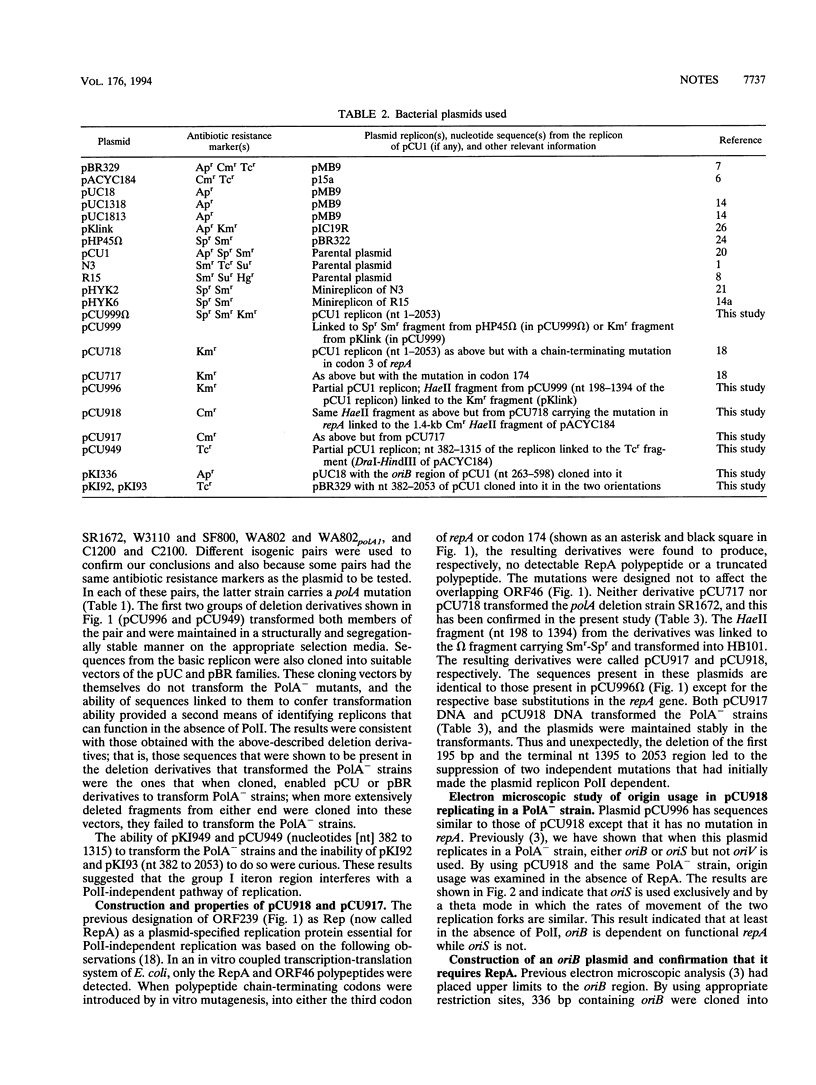
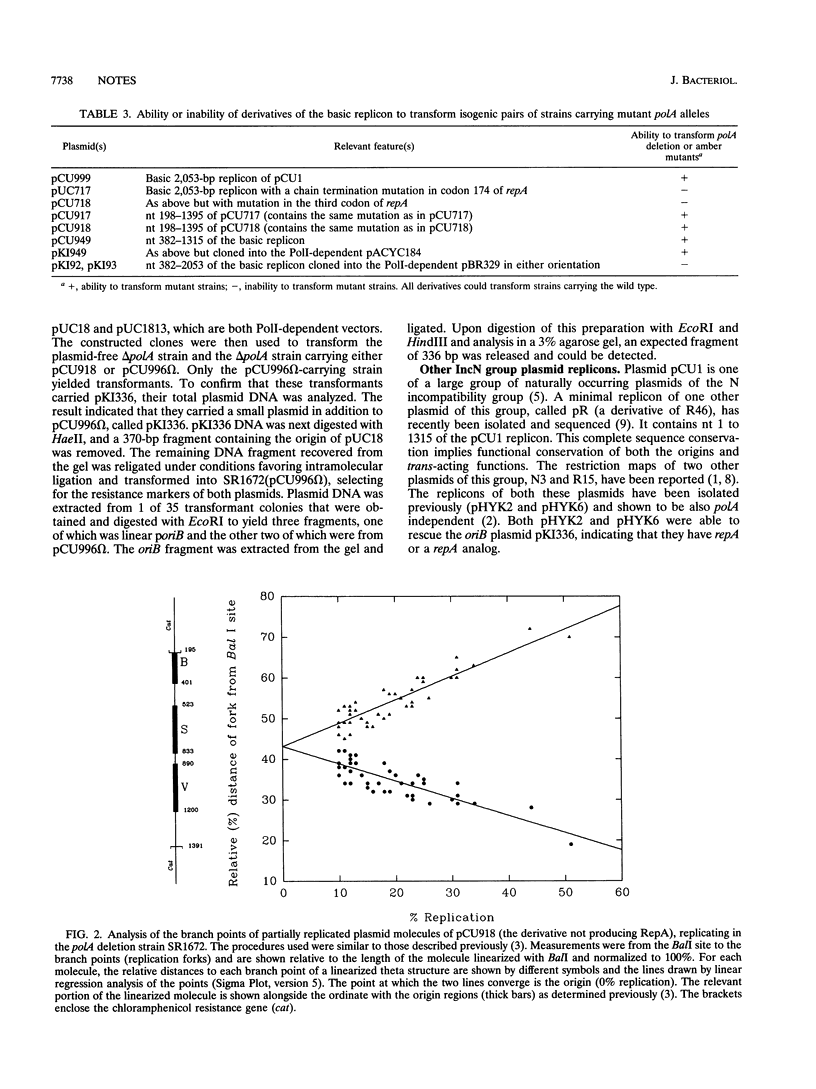
The 2,053-bp broad-host-range incompatibility group N replicon of plasmid pCU1 has two components: a region of 1,200 bp that is sufficient for its replication in Escherichia coli PolA+ and PolA- hosts and a regulatory region called the group I iteron region that contains 13 39-bp iterons. Within the 1,200-bp region, there are three replication origins, two of which, called oriB and oriS, function in PolA+ and PolA- hosts and a third, called oriV, which functions only in PolA+ hosts. The region also specifies a protein called RepA. We now show that both oriB and oriS can function in a delta polA strain but that in such a strain, only oriB has an absolute requirement for RepA. oriS can function without RepA and polymerase I provided that the iteron region is deleted and that in this circumstance, it is the only origin, the usage of which is detected. The requirements for oriB usage can thus be distinguished from those for oriS usage. The oriB region can be recovered as a plasmid only if RepA is provided in trans. These complex features of this replicon are also shown to be shared by the IncN replicons of other antibiotic resistance plasmids. Functionally distinguishable origins in a small replicon may be a way of endowing such a replicon with a broad host range.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Arai T. Genetic structure of the IncN plasmid N3. Plasmid. 1981 Nov;6(3):293–301. doi: 10.1016/0147-619x(81)90037-8. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S. K., Luck B. T., Kim H. Y., Iyer V. N. Three clustered origins of replication in a promiscuous-plasmid replicon and their differential use in a PolA+ strain and a delta PolA strain of Escherichia coli K-12. J Bacteriol. 1992 Dec;174(24):8139–8143. doi: 10.1128/jb.174.24.8139-8143.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Dobritsa A. P., Mikhailova T. G., Dubovaya V. I. Physical and genetic structure of the IncN plasmid R15. Plasmid. 1985 Sep;14(2):99–105. doi: 10.1016/0147-619x(85)90069-1. [DOI] [PubMed] [Google Scholar]
- Gigliani F., Ciotta C., Del Grosso M. F., Battaglia P. A. pR plasmid replication provides evidence that single-stranded DNA induces the SOS system in vivo. Mol Gen Genet. 1993 Apr;238(3):333–338. doi: 10.1007/BF00291991. [DOI] [PubMed] [Google Scholar]
- Grant S. G., Jessee J., Bloom F. R., Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4645–4649. doi: 10.1073/pnas.87.12.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Bedinger P., Champoux J. J., Falkow S. Deletions affecting the transposition of an antibiotic resistance gene. Proc Natl Acad Sci U S A. 1977 Feb;74(2):702–706. doi: 10.1073/pnas.74.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. W., Harnish B. W. Inversions between ribosomal RNA genes of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7069–7072. doi: 10.1073/pnas.78.11.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R., McPherson J. Hybrid pUC vectors for addition of new restriction enzyme sites to the ends of DNA fragments. Nucleic Acids Res. 1987 Mar 25;15(6):2778–2778. doi: 10.1093/nar/15.6.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C. Aspects of plasmid F maintenance in Escherichia coli. Can J Microbiol. 1988 Apr;34(4):526–535. doi: 10.1139/m88-090. [DOI] [PubMed] [Google Scholar]
- Krevolin M. D., Inman R. B., Roof D., Kahn M., Calendar R. Bacteriophage P4 DNA replication. Location of the P4 origin. J Mol Biol. 1985 Apr 20;182(4):519–527. doi: 10.1016/0022-2836(85)90238-4. [DOI] [PubMed] [Google Scholar]
- Krishnan B. R., Fobert P. R., Seitzer U., Iyer V. N. Mutations within the replicon of the IncN plasmid pCU1 that affect its Escherichia coli polA-independence but not its autonomous replication ability. Gene. 1990 Jul 2;91(1):1–7. doi: 10.1016/0378-1119(90)90155-k. [DOI] [PubMed] [Google Scholar]
- Krishnan B. R., Iyer V. N. Host Ranges of the IncN Group Plasmid pCU1 and Its Minireplicon in Gram-Negative Purple Bacteria. Appl Environ Microbiol. 1988 Sep;54(9):2273–2276. doi: 10.1128/aem.54.9.2273-2276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B. R., Iyer V. N. IncN plasmid replicon. A deletion and subcloning analysis. J Mol Biol. 1990 Jun 20;213(4):777–788. doi: 10.1016/S0022-2836(05)80263-3. [DOI] [PubMed] [Google Scholar]
- Krishnan B. R., Kim H. Y., Iyer V. N. pMUR274 and pMUR545 are not IncN group plasmids. Plasmid. 1991 Jul;26(1):78–81. doi: 10.1016/0147-619x(91)90039-y. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Prentki P., Krisch H. M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- Sharma R. C., Smith K. C. Role of DNA polymerase I in postreplication repair: a reexamination with Escherichia coli delta polA. J Bacteriol. 1987 Oct;169(10):4559–4564. doi: 10.1128/jb.169.10.4559-4564.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Crouse G. F. Construction of linker-scanning mutations using a kanamycin-resistance cassette with multiple symmetric restriction sites. Gene. 1989 Dec 7;84(1):159–164. doi: 10.1016/0378-1119(89)90150-9. [DOI] [PubMed] [Google Scholar]
- Yasukawa H., Hase T., Sakai A., Masamune Y. Rolling-circle replication of the plasmid pKYM isolated from a gram-negative bacterium. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10282–10286. doi: 10.1073/pnas.88.22.10282. [DOI] [PMC free article] [PubMed] [Google Scholar]



