Abstract
Expression of the oestrogen-regulated pNR-2/pS2 protein has been studied in paraffin sections of a series of 172 primary breast cancers using an immunohistochemical technique. Positive staining of tumour cells was found in 117 tumours (68%): most of these tumours contained only a small proportion of positive cells. pNR-2 immunohistochemical staining correlated positively and significantly with the presence of oestrogen receptor. Mean percentages of pNR-2 positive cells were lower in tumours from postmenopausal women. Smaller, better differentiated tumours were significantly more likely to stain positively for pNR-2. The percentages of pNR-2 positive tumour cells in primary tumours and synchronously excised lymph node metastases were very similar. pNR-2 expression showed an unexpected positive association with lymph node metastasis. We were unable to find any significant association between pNR-2 immunohistochemical staining and either time to relapse or overall survival. There was a significant association between pNR-2 expression in primary tumours and response to endocrine therapy on relapse: positive pNR-2 immunohistochemical staining in primary tumours is predictive of response to hormonal therapy on relapse.
Full text
PDF

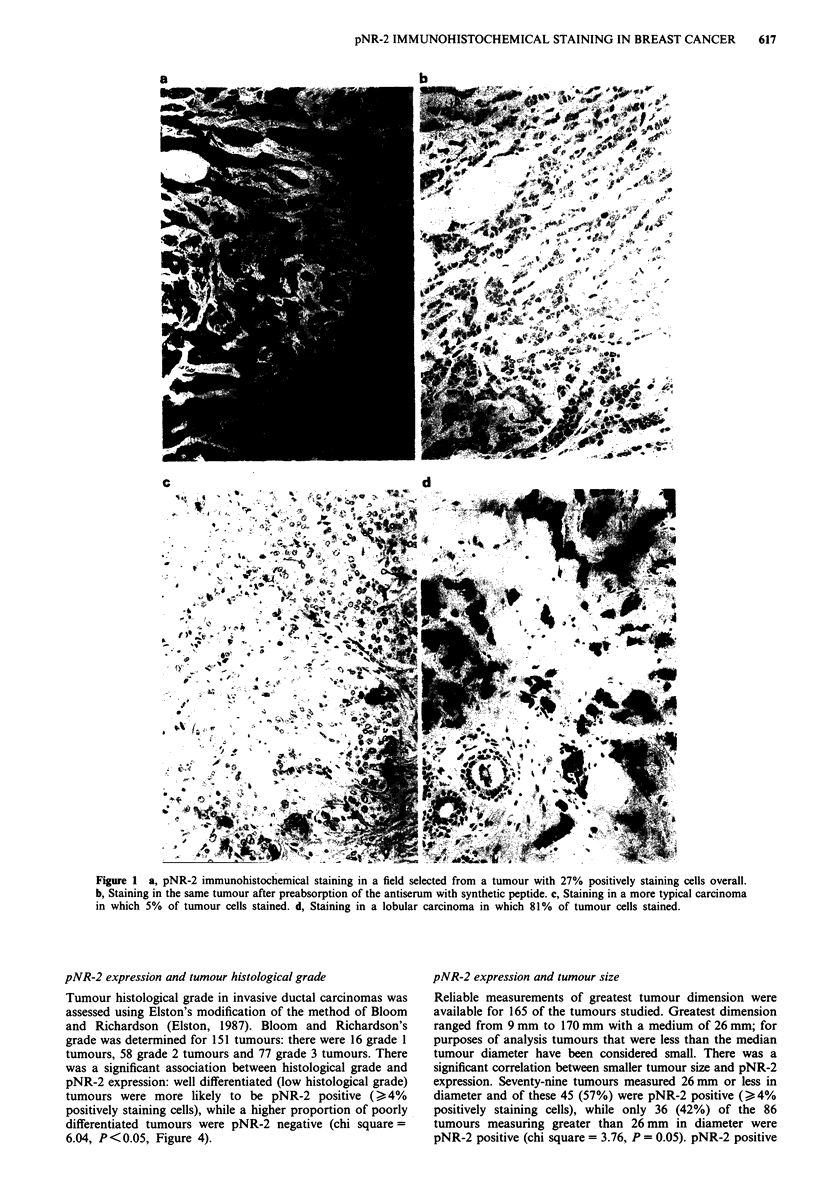
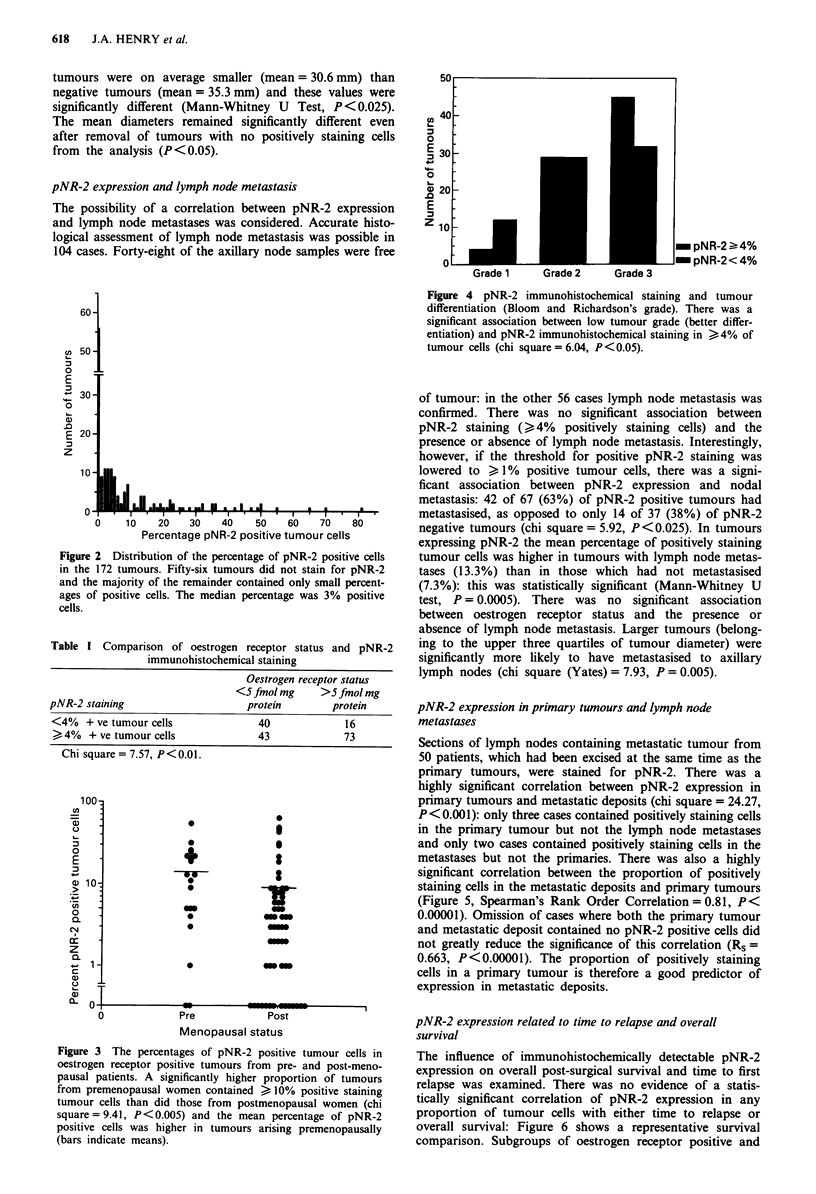




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexieva-Figusch J., Van Putten W. L., Blankenstein M. A., Blonk-Van Der Wijst J., Klijn J. G. The prognostic value and relationships of patient characteristics, estrogen and progestin receptors, and site of relapse in primary breast cancer. Cancer. 1988 Feb 15;61(4):758–768. doi: 10.1002/1097-0142(19880215)61:4<758::aid-cncr2820610421>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Berry M., Nunez A. M., Chambon P. Estrogen-responsive element of the human pS2 gene is an imperfectly palindromic sequence. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1218–1222. doi: 10.1073/pnas.86.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Jeltsch J. M., Roberts M., Chambon P. Activation of pS2 gene transcription is a primary response to estrogen in the human breast cancer cell line MCF-7. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6344–6348. doi: 10.1073/pnas.81.20.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foekens J. A., Rio M. C., Seguin P., van Putten W. L., Fauque J., Nap M., Klijn J. G., Chambon P. Prediction of relapse and survival in breast cancer patients by pS2 protein status. Cancer Res. 1990 Jul 1;50(13):3832–3837. [PubMed] [Google Scholar]
- Hawkins R. A., White G., Bundred N. J., Dixon J. M., Miller W. R., Stewart H. J., Forrest A. P. Prognostic significance of oestrogen and progestogen receptor activities in breast cancer. Br J Surg. 1987 Nov;74(11):1009–1013. doi: 10.1002/bjs.1800741118. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Nicholson S., Farndon J. R., Westley B. R., May F. E. Measurement of oestrogen receptor mRNA levels in human breast tumours. Br J Cancer. 1988 Nov;58(5):600–605. doi: 10.1038/bjc.1988.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Nicholson S., Hennessy C., Lennard T. W., May F. E., Westley B. R. Expression of the oestrogen regulated pNR-2 mRNA in human breast cancer: relation to oestrogen receptor mRNA levels and response to tamoxifen therapy. Br J Cancer. 1990 Jan;61(1):32–38. doi: 10.1038/bjc.1990.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoosein N. M., Thim L., Jørgensen K. H., Brattain M. G. Growth stimulatory effect of pancreatic spasmolytic polypeptide on cultured colon and breast tumor cells. FEBS Lett. 1989 Apr 24;247(2):303–306. doi: 10.1016/0014-5793(89)81357-2. [DOI] [PubMed] [Google Scholar]
- Jakowlew S. B., Breathnach R., Jeltsch J. M., Masiakowski P., Chambon P. Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7. Nucleic Acids Res. 1984 Mar 26;12(6):2861–2878. doi: 10.1093/nar/12.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. D., Westley B. R., May F. E. Oestrogenic activity of tamoxifen and its metabolites on gene regulation and cell proliferation in MCF-7 breast cancer cells. Br J Cancer. 1989 May;59(5):727–738. doi: 10.1038/bjc.1989.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W. J., DeSombre E. R., Jensen E. V., Greene G. L. Comparison of immunocytochemical and steroid-binding assays for estrogen receptor in human breast tumors. Cancer Res. 1985 Jan;45(1):293–304. [PubMed] [Google Scholar]
- Lippman M. E., Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature. 1975 Aug 14;256(5518):592–593. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Cloning of estrogen-regulated messenger RNA sequences from human breast cancer cells. Cancer Res. 1986 Dec;46(12 Pt 1):6034–6040. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Effects of tamoxifen and 4-hydroxytamoxifen on the pNR-1 and pNR-2 estrogen-regulated RNAs in human breast cancer cells. J Biol Chem. 1987 Nov 25;262(33):15894–15899. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Identification and characterization of estrogen-regulated RNAs in human breast cancer cells. J Biol Chem. 1988 Sep 15;263(26):12901–12908. [PubMed] [Google Scholar]
- McCarty K. S., Jr, Barton T. K., Fetter B. F., Woodard B. H., Mossler J. A., Reeves W., Daly J., Wilkinson W. E., McCarty K. S., Sr Correlation of estrogen and progesterone receptors with histologic differentiation in mammary carcinoma. Cancer. 1980 Dec 15;46(12 Suppl):2851–2858. doi: 10.1002/1097-0142(19801215)46:12+<2851::aid-cncr2820461424>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- McClelland R. A., Finlay P., Walker K. J., Nicholson D., Robertson J. F., Blamey R. W., Nicholson R. I. Automated quantitation of immunocytochemically localized estrogen receptors in human breast cancer. Cancer Res. 1990 Jun 15;50(12):3545–3550. [PubMed] [Google Scholar]
- Mouridsen H., Palshof T., Patterson J., Battersby L. Tamoxifen in advanced breast cancer. Cancer Treat Rev. 1978 Sep;5(3):131–141. doi: 10.1016/s0305-7372(78)80017-6. [DOI] [PubMed] [Google Scholar]
- Nunez A. M., Jakowlev S., Briand J. P., Gaire M., Krust A., Rio M. C., Chambon P. Characterization of the estrogen-induced pS2 protein secreted by the human breast cancer cell line MCF-7. Endocrinology. 1987 Nov;121(5):1759–1765. doi: 10.1210/endo-121-5-1759. [DOI] [PubMed] [Google Scholar]
- Osborne C. K., Yochmowitz M. G., Knight W. A., 3rd, McGuire W. L. The value of estrogen and progesterone receptors in the treatment of breast cancer. Cancer. 1980 Dec 15;46(12 Suppl):2884–2888. doi: 10.1002/1097-0142(19801215)46:12+<2884::aid-cncr2820461429>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Prud'homme J. F., Jolivet A., Pichon M. F., Savouret J. F., Milgrom E. Monoclonal antibodies against native ant denatured forms of estrogen-induced breast cancer protein (BCEI/pS2) obtained by expression in Escherichia coli. Cancer Res. 1990 Apr 15;50(8):2390–2396. [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri P. K., Simberg N., Murai J. Estrogens and breast cancer. Ann N Y Acad Sci. 1986;464:100–105. doi: 10.1111/j.1749-6632.1986.tb15997.x. [DOI] [PubMed] [Google Scholar]
- Skilton R. A., Luqmani Y. A., McClelland R. A., Coombes R. C. Characterisation of a messenger RNA selectively expressed in human breast cancer. Br J Cancer. 1989 Aug;60(2):168–175. doi: 10.1038/bjc.1989.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro S., Shousha S. Demonstration of progesterone receptors in paraffin wax sections of breast carcinoma. J Clin Pathol. 1990 Aug;43(8):671–674. doi: 10.1136/jcp.43.8.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Westley B. R., Hölzel F., May F. E. Effects of oestrogen and the antioestrogens, tamoxifen and LY117018, on four oestrogen-regulated RNAs in the EFM-19 breast cancer cell line. J Steroid Biochem. 1989 Mar;32(3):365–372. doi: 10.1016/0022-4731(89)90208-2. [DOI] [PubMed] [Google Scholar]
- Westley B. R., May F. E. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res. 1987 May 11;15(9):3773–3786. doi: 10.1093/nar/15.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- van Netten J. P., Algard F. T., Coy P., Carlyle S. J., Brigden M. L., Thornton K. R., Peter S., Fraser T., To M. P. Heterogeneous estrogen receptor levels detected via multiple microsamples from individual breast cancers. Cancer. 1985 Oct 15;56(8):2019–2024. doi: 10.1002/1097-0142(19851015)56:8<2019::aid-cncr2820560822>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]



