Abstract
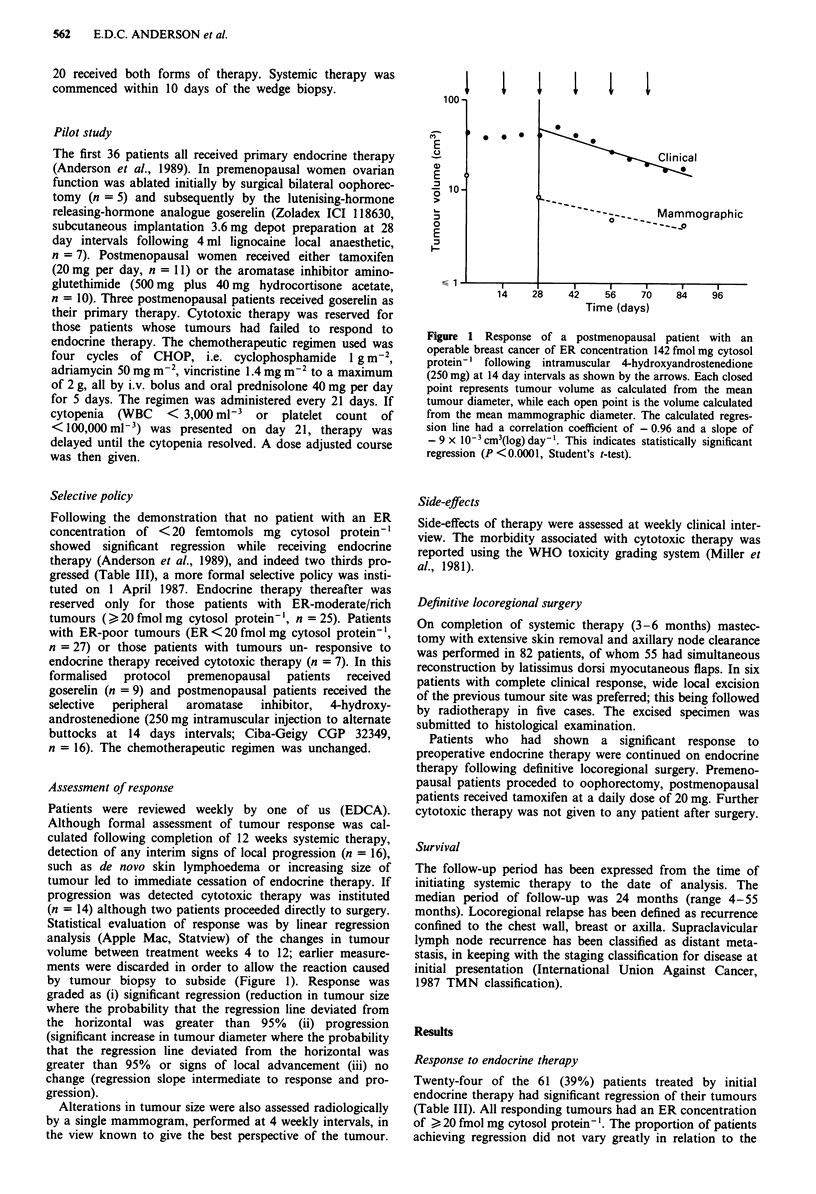
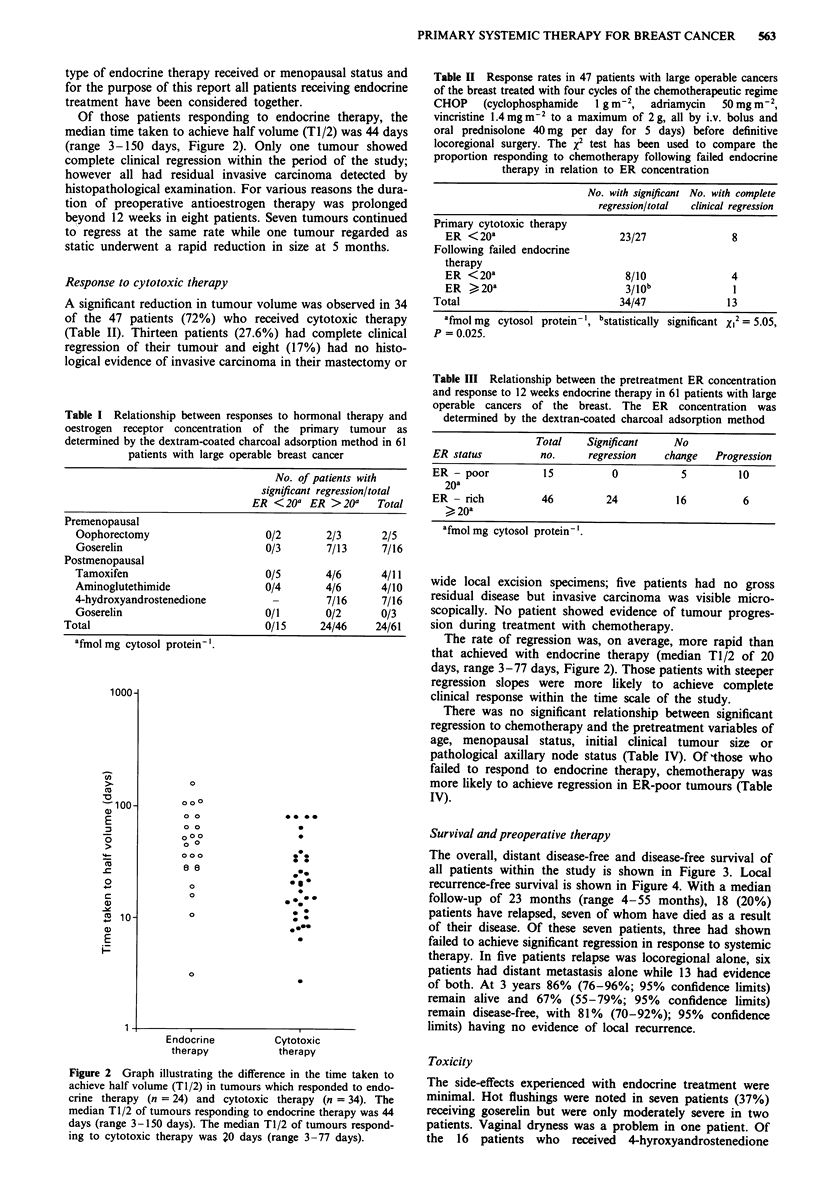
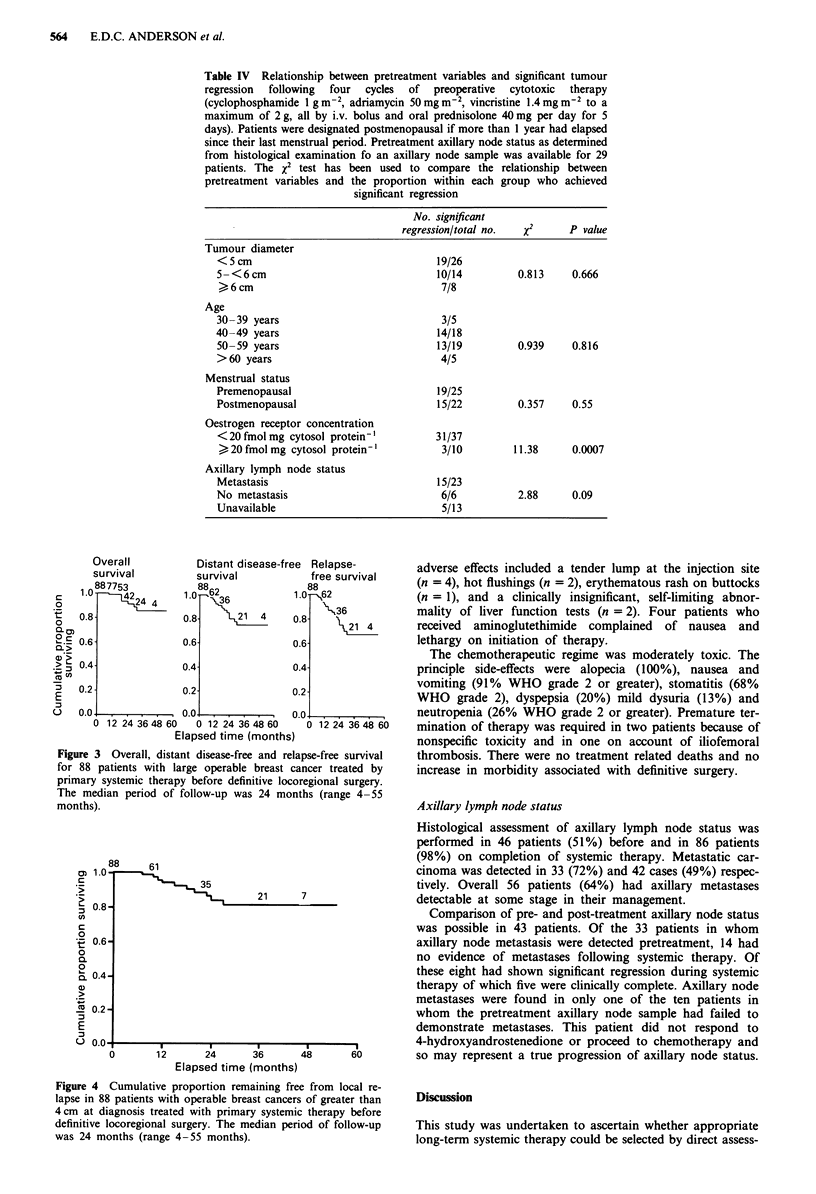
Eighty-eight patients presenting with operable breast cancer of 4 cm or greater in diameter (T2, T3, N0, N1, M0) have received primary systemic therapy. Response was assessed following 12 weeks of systemic therapy by linear regression analysis of changes in tumour volume. Definitive locoregional surgery (mastectomy n = 82, wide local excision n = 6) was performed on completion of systemic therapy (3-6 months). Response was observed in 24 (39%) of the 61 patients who received endocrine therapy; all 24 had tumours with an oestrogen receptor (ER) concentration of greater than or equal to 20 fmol mb-1 cytosol protein. Cytotoxic therapy was reserved for patients with tumours of ER concentration less than 20 fmol mg-1 cytosol protein (n = 27) or when endocrine therapy had failed (n = 20). Response was observed in 34 patients (72%). The overall survival rate at 3 years was 86%, with 81% remaining free from local relapse. We propose that the treatment policy outlined in this paper should now be tested against orthodox management by controlled randomised trial.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. D., Forrest A. P., Levack P. A., Chetty U., Hawkins R. A. Response to endocrine manipulation and oestrogen receptor concentration in large operable primary breast cancer. Br J Cancer. 1989 Aug;60(2):223–226. doi: 10.1038/bjc.1989.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A. R., De Placido S., Gallo C., Pagliarulo C., Marinelli A., Petrella G., D'Istria M., Delrio G. Adjuvant therapy with tamoxifen in operable breast cancer. 10 year results of the Naples (GUN) study. Lancet. 1988 Nov 12;2(8620):1095–1099. [PubMed] [Google Scholar]
- Carter C. L., Allen C., Henson D. E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989 Jan 1;63(1):181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Duncan W., Kerr G. R. The curability of breast cancer. Br Med J. 1976 Oct 2;2(6039):781–783. doi: 10.1136/bmj.2.6039.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B., Gunduz N., Saffer E. A. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 1983 Apr;43(4):1488–1492. [PubMed] [Google Scholar]
- Fisher B., Redmond C., Brown A., Fisher E. R., Wolmark N., Bowman D., Plotkin D., Wolter J., Bornstein R., Legault-Poisson S. Adjuvant chemotherapy with and without tamoxifen in the treatment of primary breast cancer: 5-year results from the National Surgical Adjuvant Breast and Bowel Project Trial. J Clin Oncol. 1986 Apr;4(4):459–471. doi: 10.1200/JCO.1986.4.4.459. [DOI] [PubMed] [Google Scholar]
- Forrest A. P., Levack P. A., Chetty U., Hawkins R. A., Miller W. R., Smyth J. F., Anderson T. J. A human tumour model. Lancet. 1986 Oct 11;2(8511):840–842. doi: 10.1016/s0140-6736(86)92872-2. [DOI] [PubMed] [Google Scholar]
- Glass A., Wieand H. S., Fisher B., Redmond C., Lerner H., Wolter J., Shibata H., Plotkin D., Foster R., Margolese R. Acute toxicity during adjuvant chemotherapy for breast cancer: the National Surgical Adjuvant Breast and Bowel Project (NSABP) experience from 1717 patients receiving single and multiple agents. Cancer Treat Rep. 1981 May-Jun;65(5-6):363–376. [PubMed] [Google Scholar]
- Goldie J. H., Coldman A. J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979 Nov-Dec;63(11-12):1727–1733. [PubMed] [Google Scholar]
- Hawkins R. A., Black R., Steele R. J., Dixon J. M., Forrest A. P. Oestrogen receptor concentration in primary breast cancer and axillary node metastases. Breast Cancer Res Treat. 1981;1(3):245–251. doi: 10.1007/BF01806264. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Hill A., Freedman B. A simple method for the determination of oestrogen receptor concentrations in breast tumours and other tissues. Clin Chim Acta. 1975 Oct 15;64(2):203–210. doi: 10.1016/0009-8981(75)90202-8. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A. Receptor assays and the clinical management of breast cancer. Scott Med J. 1985 Apr;30(2):73–74. doi: 10.1177/003693308503000201. [DOI] [PubMed] [Google Scholar]
- Hortobagyi G. N., Ames F. C., Buzdar A. U., Kau S. W., McNeese M. D., Paulus D., Hug V., Holmes F. A., Romsdahl M. M., Fraschini G. Management of stage III primary breast cancer with primary chemotherapy, surgery, and radiation therapy. Cancer. 1988 Dec 15;62(12):2507–2516. doi: 10.1002/1097-0142(19881215)62:12<2507::aid-cncr2820621210>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Jacquillat C., Baillet F., Weil M., Auclerc G., Housset M., Auclerc M., Sellami M., Jindani A., Thill L., Soubrane C. Results of a conservative treatment combining induction (neoadjuvant) and consolidation chemotherapy, hormonotherapy, and external and interstitial irradiation in 98 patients with locally advanced breast cancer (IIIA-IIIB). Cancer. 1988 May 15;61(10):1977–1982. doi: 10.1002/1097-0142(19880515)61:10<1977::aid-cncr2820611008>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Mansi J. L., Smith I. E., Walsh G., A'Hern R. P., Harmer C. L., Sinnett H. D., Trott P. A., Fisher C., McKinna J. A. Primary medical therapy for operable breast cancer. Eur J Cancer Clin Oncol. 1989 Nov;25(11):1623–1627. doi: 10.1016/0277-5379(89)90308-8. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Nissen-Meyer R., Host H., Kjellgren K., Mansson B., Norin T. Perioperative adjuvant chemotherapy of breast cancer: the Scandinavian experience. Recent Results Cancer Res. 1986;103:95–102. doi: 10.1007/978-3-642-82671-9_10. [DOI] [PubMed] [Google Scholar]
- Ragaz J. Preoperative (neoadjuvant) chemotherapy for breast cancer: outline of the British Columbia Trial. Recent Results Cancer Res. 1986;103:85–94. doi: 10.1007/978-3-642-82671-9_9. [DOI] [PubMed] [Google Scholar]
- SKIPPER H. E., SCHABEL F. M., Jr, WILCOX W. S. EXPERIMENTAL EVALUATION OF POTENTIAL ANTICANCER AGENTS. XIII. ON THE CRITERIA AND KINETICS ASSOCIATED WITH "CURABILITY" OF EXPERIMENTAL LEUKEMIA. Cancer Chemother Rep. 1964 Feb;35:1–111. [PubMed] [Google Scholar]
- Swain S. M., Sorace R. A., Bagley C. S., Danforth D. N., Jr, Bader J., Wesley M. N., Steinberg S. M., Lippman M. E. Neoadjuvant chemotherapy in the combined modality approach of locally advanced nonmetastatic breast cancer. Cancer Res. 1987 Jul 15;47(14):3889–3894. [PubMed] [Google Scholar]


