Abstract
Mutations in the presenilin 1 (PS-1) gene account for many cases of early-onset autosomal dominant inherited forms of Alzheimer’s disease. Recent findings suggest that PS-1 mutations may sensitize neurons to apoptosis induced by trophic factor withdrawal and exposure to amyloid β-peptide (Aβ). We now report that overexpression of the calcium-binding protein calbindin D28k prevents apoptosis in cultured neural cells expressing mutant PS-1 (L286V and M146V missense mutations). Elevations of the intracellular Ca2+ concentration and generation of reactive oxygen species induced by Aβ, and potentiated by mutant PS-1, were suppressed in calbindin-overexpressing cells. Impairment of mitochondrial function by Aβ (which preceded apoptosis) was exacerbated by PS-1 mutations and was largely prevented by calbindin. These findings suggest that PS-1 mutations render neurons vulnerable to apoptosis by a mechanism involving destabilization of cellular calcium homeostasis, which leads to oxidative stress and mitochondrial dysfunction.
Keywords: Alzheimer’s disease, amyloid, calcium binding protein, endoplasmic reticulum, mitochondrial transmembrane potential
Degeneration and death of neurons in brain regions involved in learning and memory processes underlie Alzheimer’s disease (AD), a fatal disorder that affects millions of persons worldwide. Although the cause(s) of the vast majority of cases of AD are unknown, a small percentage of cases are inherited in an autosomal dominant manner. Missense mutations in three different genes, β-amyloid precursor protein (APP; chromosome 21), presenilin 1 (PS-1; chromosome 14), and PS-2 (chromosome 1) have been causally linked to early-onset familial forms of AD (1). APP is a transmembrane protein that is the source of the 40- to 42-aa amyloid β-peptide (Aβ) that forms fibrillar aggregates (plaques) associated with degenerating neurons in the brain in AD. Several lines of evidence suggest a major role for Aβ in the neurodegenerative process in AD as follows: APP mutations result in increased production of cytotoxic forms of Aβ (2); transgenic mice expressing mutated human APP exhibit Aβ deposition and cognitive impairments (3, 4); and Aβ damages and kills cultured neurons by a mechanism involving oxidative stress and disruption of cellular calcium homeostasis (5–10). PS-1 and PS-2 encode integral membrane proteins with six or eight membrane-spanning domains (11–13) and are localized in the endoplasmic reticulum (ER; ref. 14). PSs are expressed in neurons throughout the brain (15–17) and appear to be present in both degenerating and nondegenerating neurons in AD brain (18, 19). Mutations in PS-1 account for approximately 50% of all cases of familial AD (20); understanding how PS-1 mutations promote neuron degeneration is, therefore, a critical issue in AD research.
Increased oxidative stress and disruption of neuronal calcium homeostasis appear to be interrelated final common pathways that mediate the neurodegenerative process in AD (for review, see refs. 21 and 22). There is increased protein, lipid, and DNA oxidation in association with degenerating neurons in AD (23–27). Exposure of cultured neurons to Aβ induces membrane lipid peroxidation (6, 7, 28), which mediates impairment of membrane ion-motive ATPases and glucose transporters and thereby renders neurons vulnerable to excitotoxicity and apoptosis (9, 10, 29, 30). Studies of postmortem AD brain revealed evidence for calcium-mediated proteolysis in degenerating neurons (31) and suggest an inverse relationship between expression of calcium-binding proteins in neurons and their vulnerability to death (32). Cell culture studies have shown that the mechanism of Aβ toxicity involves excessive accumulation of calcium within neurons (5, 8, 9) and that calcium influx can elicit cytoskeletal alterations similar to those seen in neurofibrillary tangles in AD (33). Expression of the calcium-binding protein calbindin D28k in cultured hippocampal neurons is correlated with increased resistance to cell death induced by a variety of insults including exposure to Aβ (34, 35).
Apoptosis is a form of cell death characterized by cell shrinkage, mitochondrial alterations, and nuclear DNA condensation and fragmentation (36–38). Neuronal apoptosis is increasingly implicated in AD based on studies of postmortem brain tissues (39, 40) and cell culture studies showing that Aβ can induce neuronal apoptosis (30, 41, 42). Recent findings suggest that PS mutations may predispose neurons to apoptotic death. Overexpression of mutant (and to a lesser extent wild type) PS-2 increased the vulnerability of pheochromocytoma (PC12) cells to apoptosis induced by trophic factor withdrawal (43, 44). Expression of mutant PS-1 in PC12 cells increased their vulnerability to apoptosis induced by Aβ and trophic factor withdrawal (45, 46). We now report that overexpression of calbindin in neural cells counteracts the proapoptotic actions of PS-1 mutations by a mechanism involving stabilization of intracellular calcium levels, suppression of oxidative stress, and preservation of mitochondrial function.
MATERIALS AND METHODS
Generation and Characterization of PC12 Cell Lines.
The PS-1 gene was amplified from a human lymphoblastoid cDNA library by PCR using 30 cycles of Expand high-fidelity PCR (Boehringer Mannheim), and the product was then cloned into the vector PCR3 (Invitrogen) yielding a vector designated PCRS182. For in vitro mutatgenesis, the KpnI–XhoI fragment from PCR182 was subcloned into pAlter-1 (Promega). The L286V or M146V mutations were generated by using mutagenic oligonucleotides according to the manufacturer’s instructions (Promega). The DNA sequence of the wild-type and mutated PS-1 genes were verified by using AmpliTaq to label the DNA with dye terminator before detection on an ABI373 DNA sequencer (Perkin–Elmer). Full-length human PS-1 cDNAs containing the L286V or M146V mutations were subcloned into pRcCMV expression vector to generate pCMV-PS1L286V and pCMV-PS1M146V. The full-length wild-type human PS-1 cDNA was subcloned into pRcCMV expression vector to generate pCMV-PS1. PC12 cells were transfected by using Lipofectamine and stable clonal transfectants from each group were selected with G418 for 4 weeks and screened for the presence of expression of the transgene by reverse transcription-coupled PCR and Western blot analysis. Because the L286V mutation creates a new PvuII restriction site and the M146V mutation destroys a BspHI restriction site in PS-1, the introduction of the mutation in the transfected cells was confirmed by reverse transcription-coupled PCR analysis. For the L286V mutation, the primers used were 5′-GTGGCTGTTTTGTGTCCGAA-3′ and 5′-GCTTCCCATTCCTCACTGAA-3′. For the M146V mutation, the primers used were 5′-TCACAGAAGATACCGAGACT-3′ and 5′-CGTTATAGGTTTTAAACACT-3′ (ref. 45 and data not shown).
PC12 cell lines stably expressing calbindin D28k were established by transfection with an expression vector containing the rat calbindin D28k cDNA subcloned into the pREP4 expression vector (Invitrogen) in which expression is under the control of Rous sarcoma virus long terminal repeat promoter. To coexpress PS-1, PS-1 L286V, or PS-1 M146V with calbindin D28k, PC12 cells overexpressing calbindin D286K were further transfected by using Lipofectamine (GIBCO/BRL) with the expression construct pCMV-PS1, pCMV-PS1 L286V, or pCMV-PS1M146V, where the expression of the transgenes were driven by enhancer-promoter sequences from the immediate-early gene of the human cytomegalovirus. Control PC12 cells were transfected with pREP4 and pRc/CMV vectors alone. Stable transfection of PC12 cells with pCMV-PS-1 or pCMV-PS1 L286V construct did not significantly affect the viability of these cells during G418 selection procedures, and the cells grew well under basal culture conditions during early passages. Transfected cells were maintained at 37°C (5% CO2/95% air atmosphere) in RPMI 1640 medium containing G418 (400 μg/ml, for PS-1- or PS-1 L286V-transfected cells) or hygromycin (400 μg/ml, for calbindin D28K-transfected cells) or both in cotransfected cell lines and supplemented 10% with heat-inactivated horse serum and 5% with heat-inactivated fetal bovine serum.
Western Blot Analysis.
Relative levels of expression of calbindin, wild-type PS-1, and PS-1L286V in the various cell lines were characterized by Western blot analysis using methods similar to those described (45, 47). Briefly, 50 μg of proteins in cell homogenates was separated by electrophoresis through a SDS/polyacrylamide gel and then transferred to a nitrocellulose sheet. After blocking with 5% milk and a 3- to 4-hr incubation in the presence of primary antibody, the nitrocellulose sheet was further processed by using horseradish peroxidase-conjugated anti-mouse secondary antibody and a chemiluminescent system (Amersham). The primary antibodies were rabbit anti-rat calbindin (1:2,000 dilution; ref. 47), rabbit anti-human PS-1 (1:200 dilution; ref. 46), and mouse mAb against α-tubulin (Sigma).
Experimental Treatments and Quantification of Apoptosis.
Immediately before experimental treatment, the medium was replaced with Locke’s solution (154 mM NaCl/5.6 mM KCl/2.3 mM CaCl2/1.0 mM MgCl2/3.6 mM NaHCO3/5 mM glucose/5 mM Hepes, pH 7.2). Synthetic Aβ1–42 was synthesized by the University of Kentucky Macromolecular Structure Facility, and stocks were prepared at a concentration of 1 mM in water and allowed to incubate for 2 hr at 37°C before addition to cultures; during this preincubation period, peptide aggregates formed as described (8, 9, 46). For analysis of apoptosis, cells were stained with the fluorescent DNA-binding dye Hoescht 33342 as described (30, 46). Cells with apoptotic nuclei (condensed and fragmented DNA) were counted in four random ×40 fields per culture; counts were made without knowledge of cell line or treatment history.
Analyses of Intracellular Calcium Levels, Oxidative Stress, and Mitochondrial Function.
Fluorescence ratio imaging of the calcium indicator dye fura-2 was performed as described (5). Cells were exposed to Aβ or vehicle for 4 hr, loaded with fura-2, and then imaged (in the continued presence of Aβ or vehicle). The ratio of the fluorescence emission at two different excitation wavelengths (334 nm and 380 nm) was used to determine the intracellular Ca2+ concentration ([Ca2+]i) as described (48). Levels of cellular oxidative stress were measured by using the fluorescent probe 2,7-dichlorofluorescin diacetate (DCF; Molecular Probes) as described (7). Briefly, cells were incubated for 50 min in the presence of 50 μM DCF, followed by washing in Hanks’ balanced saline solution containing 10 mM Hepes buffer and 10 mM glucose. Cells were imaged by using a confocal laser scanning microscope (Molecular Dynamics) coupled to an inverted microscope (Nikon). Cells were located under bright-field optics and then scanned once with the laser (488-nm excitation and 510-nm emission). Values of cellular fluorescence (average pixel intensity per cell) were obtained with the software supplied by the manufacturer (Molecular Dynamics).
Levels of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction, a measure of mitochondrial function (49) were quantified as described (30). Mitochondrial transmembrane potential was assessed by using the dye rhodamine 123 (50). Cultures were incubated for 30 min in medium, containing 5 μM rhodamine 123, and then washed with Locke’s solution. Cellular fluorescence was imaged by using a confocal laser scanning microscope with excitation at 488 nm and emission at 510 nm, and the average pixel intensity per cell was determined by using imagespace software (Molecular Dynamics).
RESULTS
PS-1 Mutations Increase Vulnerability of PC12 Cells to Aβ-Induced Apoptosis: Prevention by Calbindin.
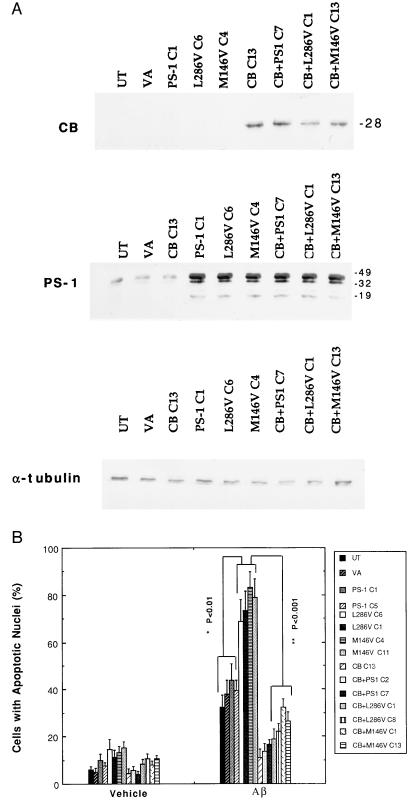
The following clonal lines of PC12 cells were used: the untransfected parent cell line, mock transfected cell lines (values for a line transfected with the empty vector pRc/CMV used for PS-1 expression, a line transfected with the empty vector pREP4 used for calbindin D28K expression, and a line transfected with both vectors were combined), two lines overexpressing wild-type PS-1 (PS-1C1 and PS1C5), two lines overexpressing the PS-1 L286V mutation (L286VC1 and L286VC6), two lines overexpressing the PS-1 M146V mutation (M146VC4 and M146VC11), a line overexpressing calbindin (CBC13), two lines overexpressing calbindin and wild-type PS-1 (CB+PS1C2 and CB+PS1C7), two lines overexpressing calbindin and the PS-1 L286V mutation (CB+L286VC1 and CB+L286VC8), and two lines overexpressing calbindin and the PS-1 M146V mutation (CB+M146VC1 and CB+M146VC13). Western blot analyses of these lines showed that levels of full-length PS-1 protein were approximately 5-fold higher in the lines transfected with wild-type or mutant human PS-1 compared with untransfected, vector-transfected, and calbindin-transfected cell lines (Fig. 1A). In addition to a 46-kDa band corresponding to full-length PS-1, immunoreactive bands of approximately 34 and 17 kDa were present. From previous findings (14), the 17-kDa band probably corresponds to a C-terminal endoproteolytic fragment of PS-1; the 34-kDa band may represent either a proteolytic product or a posttranslationally modified form of PS-1. All three bands were seen in lines overexpressing either wild-type or mutant PS-1. Levels of calbindin D28k were at or below the limit of detection in control cell lines and lines overexpressing wild-type or mutant PS-1; cell lines transfected with the pREP-CB expressed calbindin at markedly higher levels (Fig. 1A). Cell lines overexpressing PS-1, PS-1 L286V, or PS1M146V were selected based on their similar levels of overexpression of PS-1 protein, as determined by Western blot analysis (Fig. 1A).
Figure 1.
Calbindin overexpression protects PC12 cells against the proapoptotic action of mutant PS-1. (A) Representative Western blots showing levels of PS-1 and calbindin protein expression in the PC12 cell lines used. Proteins in homogenates from the indicated cell lines were separated by SDS/PAGE (50 μg of protein per lane), transferred to a nitrocellulose sheet, and immunoreacted with a polyclonal anti-calbindin antibody (Top), a polyclonal anti-PS-1 antibody (Middle), or a monoclonal anti-α-tubulin antibody (Bottom). UT, untransfected parent cell line; VA, line transfected with empty vector; PS-1C1, a line overexpressing wild-type PS-1; L286VC6, a line overexpressing the PS-1 L286V mutation; M146VC4, a line overexpressing the PS-1 M146V mutation; CB13, a line overexpressing calbindin; CB+PS1C7, a line overexpressing both wild-type PS-1 and calbindin; CB+L286VC1, a line overexpressing PS-1L286V and calbindin; and CB+M146VC12, a line overexpressing both PS-1M146V and calbindin. (B) Cultures of the indicated cell lines were exposed for 24 hr to either vehicle or 50 μM Aβ1–42. Cells were stained with Hoescht 33342, and the percentage of cells in each culture with apoptotic nuclei (condensed and fragmented DNA) was determined. Values are the mean ± SD of determinations made in four cultures (ANOVA with Scheffe’s posthoc tests).
To examine the impact of overexpression of wild-type PS-1, mutant PS-1, and/or calbindin on neural cell apoptosis, each of the cell lines was exposed for 24 hr to Aβ1–42 (50 μM) and cells with apoptotic nuclei were counted. Basal levels of apoptosis ranged from 5% to 15% among the various cell lines (Fig. 1B). In control cell lines and lines overexpressing wild-type PS-1, Aβ induced apoptosis in 33–44% of the cells (Fig. 1B). Levels of Aβ-induced apoptosis were significantly increased to 70–80% in cell lines expressing either of the PS-1 mutations. Overexpression of calbindin significantly attenuated Aβ-induced apoptosis and largely blocked the proapoptotic action of mutant PS-1 (Fig. 1B).
Aβ-Induced Elevations of [Ca2+]i and Reactive Oxygen Species Are Enhanced by Mutant PS and Blocked by Calbindin Overexpression.
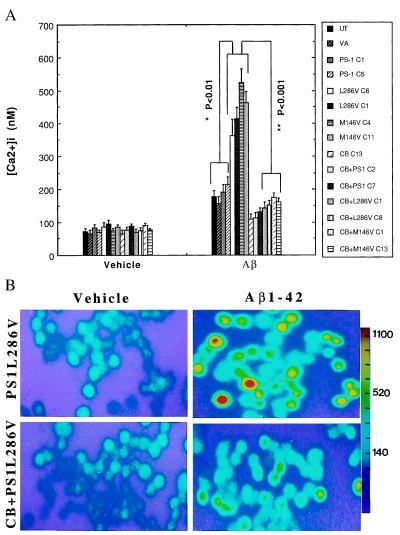
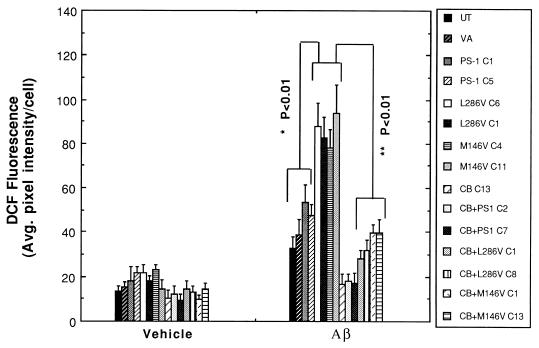
Levels of [Ca2+]i were quantified in the different cell lines 4 hr after exposure to vehicle or Aβ1–42. Basal levels of [Ca2+]i were similar in all cell lines examined, ranging from 70 to 100 nM (Fig. 2A). In control cell lines and lines overexpressing wild-type PS-1, Aβ induced increases of [Ca2+]i to levels of 150–200 nM during a 4-hr exposure period (Fig. 2). In contrast, the [Ca2+]i was increased to 350–500 nM in cells expressing either the L286V or the M146V PS-1 mutations. Overexpression of calbindin significantly attenuated Aβ-induced elevation of [Ca2+]i and completely blocked the enhanced calcium response in cells expressing mutant PS-1 (Fig. 2). Because oxidative stress is increased in cells exposed to Aβ and may play a role in Aβ-induced apoptosis (6, 7, 10, 30), we used the fluorescent probe DCF to measure relative levels of reactive oxygen species (ROS) in the various cell lines 4 hr after exposure to vehicle or Aβ1–42. In control cell lines and lines overexpressing wild-type PS-1, Aβ induced significant 3- to 4-fold increases in ROS levels (Fig. 3). The Aβ-induced increase in levels of ROS was significantly exacerbated in cells expressing either of the PS-1 mutations. In contrast, levels of ROS were not increased after exposure to Aβ in cell lines overexpressing calbindin alone or in combination with wild-type or mutant PS-1 (Fig. 3).
Figure 2.
Increases of [Ca2+]i induced by Aβ are enhanced in cells expressing mutant PS-1 and attenuated by overexpression of calbindin. (A) Cells were exposed for 4 hr to 50 μM Aβ1–42 and the [Ca2+]i in individual cells was quantified by fluorescence ratio imaging of the calcium indicator dye fura-2 (see Fig. 1 for cell lines). Values are the mean ± SD of determinations made in three cultures (50–80 cells per culture; ANOVA with Scheffe’s posthoc tests). (B) Ratio images of intracellular calcium levels in PC12 cells expressing mutant PS-1 alone (PS1L286V) or in combination with calbindin (CB+PS1L286V) 4 hr after exposure to either vehicle (water) or 50 μM Aβ1–42. The [Ca2+]i is represented on a color scale shown at the right (values are nM).
Figure 3.
Increases of cellular reactive oxygen species induced by Aβ are enhanced in cells expressing mutant PS-1 and are prevented by overexpression of calbindin. Cells were exposed for 4 hr to 50 μM Aβ1–42 and levels of ROS in individual cells were measured by using the fluorescent probe DCF (see Fig. 1 for cell lines). Values are the mean ± SD of determinations made in three cultures (40–65 cells per culture; ANOVA with Scheffe’s posthoc tests).
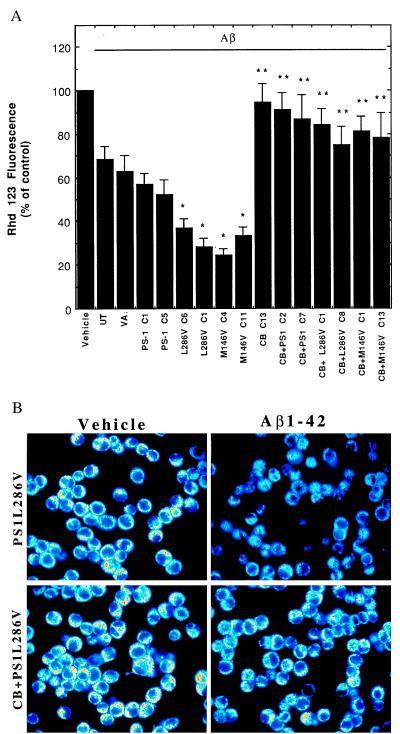
Mutant PS-1 Enhances Aβ-Induced Impairment of Mitochondrial Function: Prevention by Calbindin.
Mitochondrial alterations, including decreased energy charge/redox state and membrane depolarization, occur at relatively early stages in cells undergoing apoptosis (51, 52). Mitochondrial impairment has also been documented in brain tissues from AD patients (53), in cultured neurons exposed to Aβ (30, 50), and in fibroblasts from patients harboring PS-1 mutations (54). To determine the effects of Aβ on mitochondrial transmembrane potential, we used the fluorescent probe rhodamine 123 (50). Exposure of control PC12 cell lines, and the lines overexpressing wild-type human PS-1, to Aβ resulted in 30–40% decreases in levels of rhodamine 123 fluorescence (Fig. 4). The decrease in rhodamine 123 fluorescence induced by Aβ was significantly enhanced (greater than 60% decrease) in cells expressing either the L286V or M146V PS-1 mutations (Fig. 4). The decrease in rhodamine 123 fluorescence after Aβ exposure was largely prevented by overexpression of calbindin in the various cell lines, with the effect of calbindin being greatest in the lines expressing a mutant PS-1 (Fig. 4). Exposure of control PC12 cell lines and lines overexpressing wild-type human PS-1 to Aβ resulted in a 20–30% decrease in levels of MTT reduction during a 4-hr exposure period, indicating a decrease in electron transport activity. In parallel cultures of PC12 cells expressing mutant PS-1, Aβ caused a greater than 60% decrease in levels of MTT reduction; mitochondrial function was largely preserved in the various cell lines overexpressing calbindin alone or in combination with wild-type or mutant PS-1 (data not shown). Collectively, these findings suggest that the elevation of [Ca2+]i induced by Aβ and enhanced by PS-1 mutations played a role in impairment of mitochondrial function.
Figure 4.
Decrease in mitochondrial transmembrane potential induced by Aβ is exacerbated in cells expressing mutant PS-1s and is prevented by overexpression of calbindin. (A) Cells were exposed for 12 hr to 50 μM Aβ1–42 and levels of rhodamine 123 fluorescence were quantified (see Fig. 1 for cell lines). Values are the mean ± SD of determinations made in three cultures (ANOVA with Scheffe’s posthoc tests). ∗, P < 0.01 compared with values for untransfected, vector-transfected, and PS-1 C1 cell lines exposed to Aβ; ∗∗, P < 0.01 and ∗∗∗, P < 0.001 compared with the value for the corresponding cell line not expressing calbindin. (B) Confocal laser scanning microscope images of rhodamine 123 fluorescence in PC12 cells expressing mutant PS-1 alone (PS1L286V) or in combination with calbindin (CB+PS1L286V) 12 hr after exposure to either vehicle (water) or 50 μM Aβ1–42. Note that Aβ1–42 caused a marked decrease in rhodamine 123 fluorescence in cells lacking calbindin but not in cells overexpressing calbindin.
DISCUSSION
Our data suggest that PS-1 mutations promote an apoptotic cascade of events involving disruption of calcium homeostasis, oxidative stress, and mitochondrial dysfunction. A primary action of mutant forms of PS-1 on calcium homeostasis is indicated by the ability of calbindin overexpression to suppress Aβ-induced oxidative stress and mitochondrial dysfunction in cells expressing mutant PS-1. Similar results were obtained with two different PS-1 mutations, suggesting that a similar pathogenic mechanism may apply to all PS-1 mutations. Increased [Ca2+]i and oxygen radical production may both contribute to the proapoptotic action of PS-1 mutations because agents that block Ca2+ release from ER or influx through plasma membrane channels and antioxidants, such as vitamin E, can protect PC12 cells overexpressing mutant PS-1 against cell death induced by Aβ or trophic factor withdrawal (45, 46). The specific mechanism whereby mutant PS-1 disrupts cellular calcium homeostasis is unknown but may involve an effect on ER calcium regulation, as suggested by the localization of PS-1 in the ER (14) and the demonstrations of enhanced calcium responses to agonists that induce calcium release from ER in PC12 cells expressing mutant PS-1 (45) and in fibroblasts from human carriers of PS-1 mutations (55). Indeed, calcium release from ER induced by the ER Ca2+-ATPase inhibitor thapsigargin is sufficient to trigger apoptosis in several types of cells (56, 57).
Immunohistochemical analyses of postmortem human brain and spinal cord tissue have suggested that neurons expressing high levels of calbindin are relatively resistant to death in several prominent neurodegenerative conditions including AD (58), Parkinson’s disease (59), Down’s syndrome (60), Huntington’s disease (61), and amyotrophic lateral sclerosis (62). Calbindin-immunoreactive neurons are also spared in hippocampus from human patients with severe temporal lobe epilepsy and in rats subjected to kainate-induced seizures (63). Interestingly, levels of calbindin expression in neurons in the brain may decrease with normal aging (64), a change that would be expected to render the neurons susceptible to a variety of insults and disease states. Cell culture studies have shown that hippocampal neurons expressing calbindin are relatively resistant to death induced by exposure to excitatory amino acids (34) and Aβ (35). The present data show that overexpression of calbindin in PC12 cells results in a marked attenuation of the increase of [Ca2+]i induced by Aβ. Further evidence for a neuroprotective role for calbindin comes from studies showing that calbindin expression can be induced by brain injury (47, 65) and that trophic factors that induce calbindin expression in cultured hippocampal neurons (e.g., basic fibroblast growth factor and tumor necrosis factor) also protect those neurons against excitotoxic and metabolic insults (66–68). Calbindin, which is localized primarily in the cytosol and ER (69, 70), is thought to prevent sustained elevations of [Ca2+]i by acting as a calcium buffer (71).
An increasing amount of data suggest that apoptosis may be the predominant form of neuronal death in AD. Several laboratories have provided evidence that DNA damage and morphological changes in neurons in AD brain that are consistent with apoptosis (39, 40). In addition, altered expression of apoptosis-related genes such as c-jun and bcl-2 family members have been documented in vulnerable neurons in AD brain (72). Previous work in our laboratory (45, 46) and other laboratories (43) has shown that PS mutations can increase vulnerability of cultured cells to apoptosis induced by trophic factor withdrawal and exposure to Aβ. Deng et al. (44) and Wolozin and associates (43) provided evidence that overexpression of wild-type and mutant PS-2 can induce apoptosis in the absence of an apoptotic insult. We have not observed spontaneous apoptosis in PC12 cell lines stably overexpressing either wild-type or mutant PS-1. This indicates that the effect of overexpression of PS-1 may be different from that of PS-2 and is consistent with the observation that overexpression of wild-type or mutant PS-1 does not result in any apparent neuronal death under basal conditions in transgenic mice (73). However, we cannot rule out the possibility that lines overexpressing very high levels of mutant PS-1 died during the selection process. Our data suggest that mutant PS-1 may promote neuronal apoptosis in AD by perturbing cellular calcium homeostasis. Additional data support a proapoptotic role for PS mutations, including recent studies showing that caspase-mediated cleavage of PSs is enhanced in cells expressing mutant PSs (74) and that PS mutations alter APP processing in a manner that increases production of neurotoxic Aβ and decreases levels of neuroprotective secreted forms of APP (75, 76).
Previous studies have shown that increases of [Ca2+]i can induce oxidative stress, which may result from an adverse effect of sustained elevations of [Ca2+]i on mitochondrial electron transport leading to superoxide anion radical production and its conversion to hydrogen peroxide and peroxynitrite (77–79). We found that Aβ induced an increase in DCF fluorescence in PC12 cells that was significantly enhanced in cells expressing mutant PS-1. It should be noted that although the increased DCF fluorescence likely reflects hydrogen peroxide accumulation (7, 50, 77), DCF can be oxidized by other ROS including peroxynitrite. Calbindin overexpression largely suppressed the Aβ-induced increase in ROS production and blocked the adverse effect of mutant PS-1 on ROS accumulation. These findings suggest that PS-1 mutations may promote cellular oxidative stress by disrupting calcium homeostasis. Consistent with the latter possibility, we have found that dantrolene (an agent that blocks calcium release from the ER) can block Aβ-induced increases in ROS levels in cells expressing mutant PS-1 (Q.G. and M.P.M., unpublished data). Other studies have shown that increases in ROS levels occur early in the process of thapsigargin-induced apoptosis of thymocytes (80). Antioxidants and Bcl-2 appear to act downstream of elevation of [Ca2+]i in protecting cells against thapsigargin-induced apoptosis (81). Consistent with a role for enhanced oxidative stress in the proapoptotic action of mutant PS-1, Bcl-2 protected PC12 cells expressing mutant PS-1 against apoptosis induced by trophic factor withdrawal (46).
Mitochondrial impairment after exposure of PC12 cells to Aβ, as indicated by decreases in levels of MTT reduction and rhodamine 123 fluorescence, was exacerbated in cells expressing mutant PS-1; calbindin prevented this adverse effect of mutant PS-1. Decreases in levels of MTT reduction occur early in cells undergoing apoptosis in response to many different insults including exposure to Aβ (30, 50, 79). Additonal mitochondrial alterations linked to apoptosis include loss of mitochondrial transmembrane potential, formation of permeability transition pores, and release of proteins that trigger nuclear apoptosis such as cytochrome c and an apoptosis-inducing factor (38). We found that loss of mitochondrial transmembrane potential after exposure to Aβ was significantly exacerbated in PC12 cells expressing mutant PS-1 and that calbindin counteracted the adverse effect of mutant PS-1. These findings suggest that elevated [Ca2+]i mediates apoptotic mitochondrial alterations induced by Aβ and their potentiation by PS-1 mutations. Our data are consistent with previous studies suggesting a mechanistic link between ER calcium release and mitochondrial alterations involved in apoptosis. For example, thapsigargin can induce mitochondrial permeability transition (82), and agents that block the permeability transition (e.g., cyclosporin A) can prevent thapsigargin-induced apoptosis (83). A more detailed understanding of the mechanism whereby PS-1 modulates neuronal calcium homeostasis and cell survival may reveal molecular targets upon which to aim antiapoptotic therapeutic approaches in AD.
Acknowledgments
We thank W. Fu, H. Luo, and J. Xie for technical assistance and B. Sopher for providing plasmids containing wild-type and mutant PS-1 cDNAs. This work was supported by grants to M.P.M. from the National Institutes of Health (AG14554, AG05144, NS35253, and AG10836).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: Aβ, amyloid β-peptide; AD, Alzheimer’s disease; APP, β-amyloid precursor protein; PS, presenilin; [Ca2+]i, intracellular Ca2+ concentration; ER, endoplasmic reticulum; DCF, 2,7-dichlorofluorescin diacetate; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; ROS, reactive oxygen species.
References
- 1.Hardy J. Trends Neurosci. 1997;20:154–159. doi: 10.1016/s0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 2.Yankner B A. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 3.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 4.Games D, Adams D, Alessandrinl R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. J Neurosci. 1992;12:376–389. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 5.Mattson M P. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 6.Behl C, Davis J B, Lesley R, Schubert D. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 7.Goodman Y, Mattson M P. Exp Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- 8.Mattson M P, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel R E. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattson M P, Tomaselli K, Rydel R E. Brain Res. 1993;621:35–49. doi: 10.1016/0006-8993(93)90295-x. [DOI] [PubMed] [Google Scholar]
- 10.Mark R J, Hensley K, Butterfield D A, Mattson M P. J Neurosci. 1995;15:6239–6249. doi: 10.1523/JNEUROSCI.15-09-06239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doan A, Thinakaran G, Borchelt D R, Slunt H H, Ratovistsky T, Podlisny M, Selkoe D J, Seeger M, Gandy S E, Price D L, Sisodia S S. Neuron. 1996;17:1023–1030. doi: 10.1016/s0896-6273(00)80232-9. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Greenwald I. Neuron. 1996;17:1015–1021. doi: 10.1016/s0896-6273(00)80231-7. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann S, Chiesa R, Harris D A. J Biol Chem. 1997;272:12047–12051. doi: 10.1074/jbc.272.18.12047. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs D M, Fausett H J, Page K J, Kim T-W, Moir R D, Merriam D E, Hollister R D, Hallmark O G, Mancini R, Felsenstein K M, et al. Nat Med. 1996;2:224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- 15.Cook D B, Sung J C, Golde T E, Felsenstein K M, Wojczyk B S, Tanzi R E, Trojanowski J, Lee V, Doms R. Proc Natl Acad Sci USA. 1996;93:9223–9228. doi: 10.1073/pnas.93.17.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elder G A, Tezapsidis N, Carter J, Shioi J, Bouras C, Li D, Johnston J M, Efthimiopoulos S, Friedrich V L, Robakis N K. J Neurosci Res. 1996;45:308–320. doi: 10.1002/(SICI)1097-4547(19960801)45:3<308::AID-JNR13>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Cribbs D, Chen L, Bendle S, La Ferla F M. Am J Pathol. 1996;148:1797–1806. [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy G M, Forno L S, Ellis W G, Nochlin D, Levy-Lahad E, Poorkaj P, Bird T D, Jiang Z, Cordell B. Am J Pathol. 1996;149:1839–1846. [PMC free article] [PubMed] [Google Scholar]
- 19.Giannakopoulos P, Bouras C, Kovari E, Shioi J, Tezapsidis N, Hof P R, Robakis N K. Am J Pathol. 1997;150:429–436. [PMC free article] [PubMed] [Google Scholar]
- 20.Sherrington R, Rogaev E I, Liang Y, Rogaeva E A, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. Nature (London) 1995;375:754–760. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 21.Mattson M P, Mark R J, Furukawa K. Chem Res Toxicol. 1997;10:507–517. doi: 10.1021/tx9601317. [DOI] [PubMed] [Google Scholar]
- 22.Smith M A, Sayre L M, Monnier V M, Perry G. Trends Neurosci. 1995;18:172–176. doi: 10.1016/0166-2236(95)93897-7. [DOI] [PubMed] [Google Scholar]
- 23.Smith C D, Carney J M, Starke-Reed P E, Oliver C N, Stadtman E R, Floyd R A, Markesbery W R. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovell M A, Ehmann W D, Butler S M, Markesbery W R. Neurology. 1995;45:1594–1601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 25.Mecocci P, Macgarvey U, Beal M F. Ann Neurol. 1994;36:102–106. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 26.Sayre L M, Zelasko D A, Harris P L R, Perry G, Salomon R G, Smith M A. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 27.Lovell M A, Mattson M P, Markesbery W R. Neurobiol Aging. 1997;18:457–461. doi: 10.1016/s0197-4580(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 28.Butterfield D A, Hensley K, Harris M, Mattson M P, Carney J. Biochem Biophys Res Commun. 1994;200:710–715. doi: 10.1006/bbrc.1994.1508. [DOI] [PubMed] [Google Scholar]
- 29.Mark R J, Pang Z, Geddes J W, Uchida K, Mattson M P. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruman I, Bruce-Keller A J, Bredesen D E, Waeg G, Mattson M P. J Neurosci. 1997;17:5089–5100. doi: 10.1523/JNEUROSCI.17-13-05089.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nixon R A, Saito K I, Grynspan F, Griffin W R, Katayama S, Honda T, Mohan P S, Shea T B, Beermann M. Ann NY Acad Sci. 1994;747:77–91. doi: 10.1111/j.1749-6632.1994.tb44402.x. [DOI] [PubMed] [Google Scholar]
- 32.Iacopino A M, Quintero E M, Miller E K. Neurodegeneration. 1994;3:1–20. [Google Scholar]
- 33.Mattson M P. Neuron. 1990;4:105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- 34.Mattson M P, Rychlik B, Chu C, Christakos S. Neuron. 1991;6:41–51. doi: 10.1016/0896-6273(91)90120-o. [DOI] [PubMed] [Google Scholar]
- 35.Prehn J H, Bindokas V P, Jordan J, Galindo M F, Ghadge G D, Roos R, Boise L H, Thompson C B, Krajewski S, Reed J C. Mol Pharmacol. 1996;49:319–328. [PubMed] [Google Scholar]
- 36.Bredesen D E. Ann Neurol. 1995;38:839–851. doi: 10.1002/ana.410380604. [DOI] [PubMed] [Google Scholar]
- 37.Steller H. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 38.Kroemer G, Zamzami N, Susin S. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 39.Su J H, Anderson A J, Cummings B, Cotman C W. NeuroReport. 1994;5:2529–2533. doi: 10.1097/00001756-199412000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Smale G, Nichols N R, Brady D R. Exp Neurol. 1995;133:225–230. doi: 10.1006/exnr.1995.1025. [DOI] [PubMed] [Google Scholar]
- 41.Forloni G, Chiesa R, Smiroldo S, Verga L. NeuroReport. 1993;4:523–526. doi: 10.1097/00001756-199305000-00015. [DOI] [PubMed] [Google Scholar]
- 42.Loo D, Copani A, Pike C, Whittemore E, Walencewicz A, Cotman C W. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolozin B, Iwasaki K, Vito P, Ganjei J K, Lacana E, Sunderland T, Zhao B, Kusiak J W, Wasco W, D’Adamio L. Science. 1996;274:1710–1713. doi: 10.1126/science.274.5293.1710. [DOI] [PubMed] [Google Scholar]
- 44.Deng G, Pike C J, Cotman C W. FEBS Lett. 1996;397:50–54. doi: 10.1016/s0014-5793(96)01142-8. [DOI] [PubMed] [Google Scholar]
- 45.Guo Q, Furukawa K, Sopher B L, Pham D G, Xie J, Robinson N, Martin G M, Mattson M P. NeuroReport. 1996;8:379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 46.Guo G, Sopher B L, Pham D G, Furukawa K, Robinson N, Martin G M, Mattson M P. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattson M P, Cheng B, Baldwin S A, Smith-Swintosky V L, Keller J, Geddes J W, Scheff S W, Christakos S. J Neurosci Res. 1995;42:357–370. doi: 10.1002/jnr.490420310. [DOI] [PubMed] [Google Scholar]
- 48.Grynkiewicz G, Poenie M, Tsien R. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 49.Mosmann T. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 50.Johnson L V, Walsh M L, Bokus B J, Chen L B. J Cell Biol. 1981;88:526–532. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zamzami N, Susin S A, Marchetti P, Hirsch T, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marchetti P, Castedo M, Susin S A, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blass J P. Hippocampus. 1993;3:45–54. [PubMed] [Google Scholar]
- 54.Sheu K-F R, Cooper A J L, Koike D, Koike M, Lindsay D, Blass J P. Ann Neurol. 1994;35:312–318. doi: 10.1002/ana.410350311. [DOI] [PubMed] [Google Scholar]
- 55.Ito E O, Etcheberrigaray R, Nelson T J, McPhie L, Tofel-Grehl B, Gibson G E, Alkon D L. Proc Natl Acad Sci USA. 1994;91:534–38. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaneko Y, Tsukamoto A. Cancer Lett. 1994;97:147–155. doi: 10.1016/0304-3835(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 57.Muthukkumar S, Nair P, Sells S F, Maddiwar N G, Jacob R J, Rangnekar V M. Mol Cell Biol. 1995;15:6262–6272. doi: 10.1128/mcb.15.11.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hof P, Morrison J. Exp Neurol. 1991;111:293–301. doi: 10.1016/0014-4886(91)90096-u. [DOI] [PubMed] [Google Scholar]
- 59.Yamada T, McGeer P L, Baimbridge K G, McGeer E G. Brain Res. 1990;526:303–307. doi: 10.1016/0006-8993(90)91236-a. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi K, Emson P C, Mountjoy C Q, Thornton S N, Lawson E M, Mann D M A. Neurosci Lett. 1990;113:17–22. doi: 10.1016/0304-3940(90)90487-t. [DOI] [PubMed] [Google Scholar]
- 61.Ferrante J R, Kowall W N, Richardson J P E. J Neurosci. 1991;11:3877–3887. doi: 10.1523/JNEUROSCI.11-12-03877.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alexianu M E, Ho B K, Mohamed A H, La Bella V, Smith R G, Appel S H. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- 63.Sloviter R S. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- 64.Iacopino A M, Christakos S. Proc Natl Acad Sci USA. 1990;87:4078–4082. doi: 10.1073/pnas.87.11.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lowenstein D H, Gwinn R P, Seren M S, Simon R P, McIntosh T K. Mol Brain Res. 1994;22:299–308. doi: 10.1016/0169-328x(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 66.Cheng B, Mattson M P. Neuron. 1991;7:1031–1041. doi: 10.1016/0896-6273(91)90347-3. [DOI] [PubMed] [Google Scholar]
- 67.Collazo D, Takahashi H, McKay R D G. Neuron. 1992;9:643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- 68.Cheng B, Christakos S, Mattson M P. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 69.DiFiglia M, Christakos S, Aronin N. J Comp Neurol. 1989;279:653–665. doi: 10.1002/cne.902790411. [DOI] [PubMed] [Google Scholar]
- 70.Pickel V M, Heras A. Neuroscience. 1996;71:167–178. doi: 10.1016/0306-4522(95)00441-6. [DOI] [PubMed] [Google Scholar]
- 71.Chard P S, Bleakman D, Christakos S, Fullmer C S, Miller R J. J Physiol. 1993;472:341–357. doi: 10.1113/jphysiol.1993.sp019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson A J, Su J H, Cotman C W. J Neurosci. 1996;16:1710–1719. doi: 10.1523/JNEUROSCI.16-05-01710.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duff K, Eckman C, Zehr C, Yu X, Prada C-M, Perez-Tur J, Hutton M, Buee L, Harigaya Y, Yager D, et al. Nature (London) 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 74.Kim T-W, Pettingell W H, Jung Y-K, Kovacs D M, Tanzi R E. Science. 1997;277:373–376. doi: 10.1126/science.277.5324.373. [DOI] [PubMed] [Google Scholar]
- 75.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird T D, Hardy J, Hutton M, Kukull W, et al. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 76.Furukawa K, Sopher B, Rydel R E, Begley J G, Martin G M, Mattson M P. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 77.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. Nature (London) 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 78.Mattson M P, Lovell M A, Furukawa K, Markesbery W R. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- 79.Kruman I, Guo Q, Mattson M P. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 80.Bustamante J, Tovar B A, Montero G, Boveris A. Arch Biochem Biophys. 1997;337:121–128. doi: 10.1006/abbi.1996.9754. [DOI] [PubMed] [Google Scholar]
- 81.Distelhorst C W, McCormick T S. Cell Calcium. 1996;19:473–483. doi: 10.1016/s0143-4160(96)90056-1. [DOI] [PubMed] [Google Scholar]
- 82.Hoek J B, Farber J L, Thomas A P, Wang X. Biochim Biophys Acta. 1995;1271:93–102. doi: 10.1016/0925-4439(95)00015-v. [DOI] [PubMed] [Google Scholar]
- 83.Waring P, Beaver J. Exp Cell Res. 1996;227:264–276. doi: 10.1006/excr.1996.0276. [DOI] [PubMed] [Google Scholar]