Abstract
Background
Chordoid meningioma is a rare meningioma variant characterised by epithelioid cord‐like tumour cells in a myxoid stroma. It is classified as grade II (World Health Organization) tumours, as they have a tendency to behave more aggressively than traditional meningiomas and have a greater likelihood of recurrence.
Aims
To report the features of intraoperative imprint smears of five cases of chordoid meningioma.
Methods
The intraoperative squash smears were reviewed for cellularity, cellular atypia, mitotic figure, cytoplasmic vacuolation, intranuclear inclusion, presence of a cohesive cord of tumour cells, whorl‐like structure, psammoma bodies, chronic inflammatory cells (lymphocytes and plasma cells), background mucin and necrosis.
Results
All cases were of moderate to high cellularity, with cohesive cords of bland tumour cells possessing uniformly round nuclei with smooth nuclear outline, stippled chromatin and small nucleoli, with cytoplasmic vacuolation and chronic inflammatory cells in the background. Intranuclear inclusions (80%) and whorl‐like structures (60%) were also common. Necrotic background, psammoma bodies or mitotic figures were consistently absent.
Conclusions
The cytological features of chordoid mengiomas are distinctive, and intraoperative imprint diagnosis is feasible.
Chordoid meningioma is a rare variant of meningioma characterised by epithelioid cord‐like tumour cells in a myxoid stroma. It is classified as grade II (World Health Organization (WHO)) tumours, as they have a tendency to behave more aggressively than traditional meningiomas and have a greater likelihood of recurrence. Five cases of chordoid meningiomas were retrieved. The clinical features, intraoperative imprint smears and subsequent histological sections showed distinctive and characteristic findings.
Materials and methods
Five cases with chordoid meningioma with intraoperative squash smears and confirmed by subsequent formalin‐fixed paraffin‐wax‐embedded sections were identified from the archives of the Department of Pathology, Kwong Wah Hospital, Kowloon, Hong Kong (n = 2); Princess Margaret Hospital, Kwai Chung, Hong Kong (n = 2); and Prince of Wales Hospital, Shatin, Hong Kong (n = 1) over a period of 5 years. The clinical information, presentation, treatment and follow‐up were retrieved.
Squash smears were made from the intraoperative frozen section material and stained with H&E stain. The remaining materials and the follow‐up specimens were fixed in formalin and embedded in paraffin wax.
In all cases, the following components were assessed from the smear: cellularity, cellular atypia, mitotic figure, cytoplasmic vacuolation, intranuclear inclusion, presence of a cohesive cord of tumour cells, whorl‐like structure, psammoma bodies, chronic inflammatory cells (lymphocytes and plasma cells), background mucin and necrosis. Cellular atypia was defined as tumour cells with high nucleocytoplasmic ratio, with or without prominent nucleoli. All the other cytomorphological parameters were taken as present or absent in a qualitative manner. The proportion of the chordoid component within the tumour was also assessed in the histological section.
Pathology and results
In our cases, the patients were adults with an age range of 40–72 years (mean 56.4 years). Three patients were male and two were female. The main symptoms were attributed to the mass effect of the tumour. Table 1 summarises the other clinical information.
Table 1 Clinical information.
| Case number | Sex/age | Site of tumour | Presentation | Imaging findings | Blood tests | Operation | Follow‐up |
|---|---|---|---|---|---|---|---|
| Case 1 | F/40 | Right lateral sphenoid wing (primary); right orbit (recurrence) | Right eye proptosis | MRI: a homogeneously enhancing right intraorbital mass of greatest dimension 4.5 cm×2 cm×1.3 cm, with compression on the globe and optic nerve | Only normochromic normocytic anaemia. No other haematological abnormality noted | Craniectomy, orbitectomy, removal of the superolateral aspect of the right orbit, excision of orbital tumour, as well as excision of involved dura | Neurologically stable with normal vision and full range of extraocular movement |
| Case 2 | M/72 | Right frontoparietal region | Sudden collapse | MRI: a large 8.5 cm×6 cm×6 cm mass lesion occupying almost the whole right middle cranial fossa with midline shift. The mass is basically extra‐axial | No haematological abnormality noted | Craniectomy and excision of tumour and involved dura | Neurologically stable. Only encephalomalacia with compensatory increase in ventricular size |
| Case 3 | F/40 | Left sphenoidal ridge | Headache | MRI: a tumour measuring 9 cm×7 cm×5 cm and touches onto left body and wings of the sphenoid as well as the basalfrontal bone (orbital roof) peripherally. This is an extra‐axial lesion and causes a midline shift and mild hydrocephalus | No haematological abnormality noted | Craniectomy and excision of tumour and involved dura | Neurologically stable. Referred for radiotherapy |
| Case 4 | M/68 | Olfactory groove | Decreased visual acuity and olfactory function | CT: hyperdense space‐occupying lesion 4.7×4.2×3.5 cm, around the anterior sagittal falx and the anterior cranial floor. The lesion appears to have a broad base against the anterior cranial floor, with specks of calcifications within. Significant perilesional vasogenic oedema is present in bilateral frontal and right temporal lobes | No haematological abnormality noted | Right orbitozygomatic craniectomy with subtotal resection of anterior skull base meningioma | Neurologically stable. Referred for radiotherapy |
| Case 5 | M/62 | Left cerebellum | Vertigo | CT: a 5 cm calcified lesion with surrounding oedema and cystic lesion in the left cerebellum, associated with obstructive hydrocephalus | No haematological abnormality noted | Left suboccipital craniectomy, extraventricular drainage and subtotal tumour resection | Neurologically stable. Referred for radiotherapy. Stroke 2 years after the surgery |
CT, Computed tomography; MRI, magnetic resonance imaging.
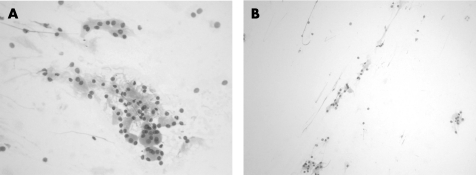
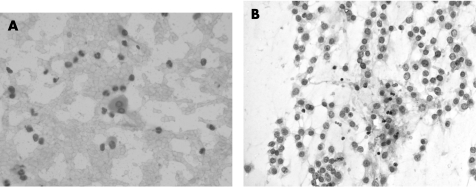
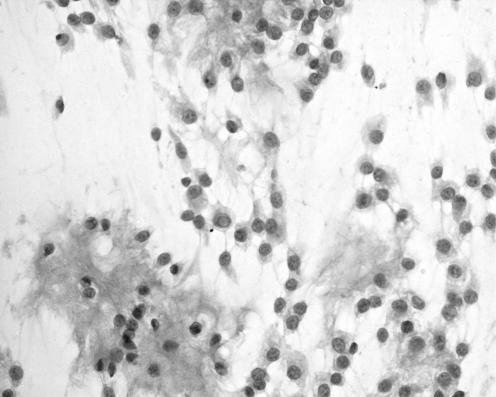
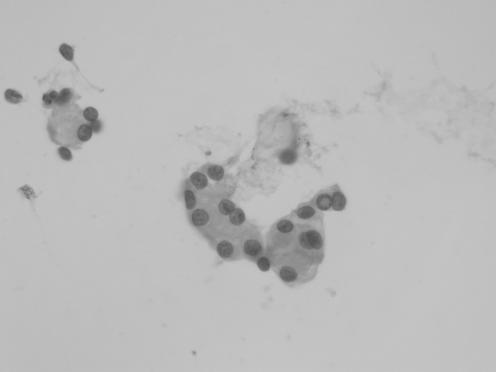
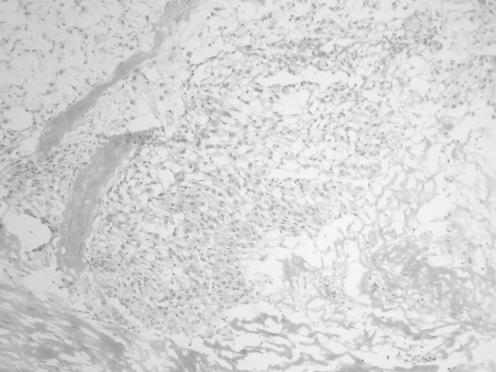
The cytological preparations in three cases showed moderate cellularity (cases 1, 2 and 5) and the remaining two cases were of high cellularity (cases 3 and 4). In all cases, there were cohesive cords of tumour cells and cytoplasmic vacuolation (fig 1). Chronic inflammatory cells were consistently present in the background or admixed with the tumour cell clusters (fig 3). Intranuclear inclusion was noted in four (80%) cases (cases 1, 2, 4 and 5). Whorl‐like structure was identified in three cases (cases 3, 4 and 5). In two cases (cases 3 and 5), there was mucin material in the background (fig 4). In all cases, there was no cellular atypia and the tumour cells possessed uniformly round nuclei, with smooth nuclear outline, stippled chromatin, small nucleoli, intranuclear inclusions and indistinct cytoplasmic borders (fig 2). Necrotic background, psammoma bodies or mitotic figures were consistently absent.
Figure 1 (A) Case 1, H&E, ×100; (B) Case 2, H&E, ×100. Clusters of tumour cells arrange in cohesive cords.
Figure 3 (A) Isolated tumour cell sets in a background of neutrophils and lymphocytes (case 1), H&E, ×400. (B) Scattered lymphocytes are admixed with cords of tumour cells (case 5), H&E, ×200.
Figure 4 Cords and small clusters of tumour cells in a mucoid background. Some tumour cells show intranuclear inclusion and cytoplasmic vacuolation (case 5), H&E, ×200.
Figure 2 Tumour cells with typical cytological features such as uniform nuclei with smooth nuclear outline, stippled chromatin, small nucleoli, intranuclear inclusions and indistinct cytoplasmic border (case 1), H&E, ×200.
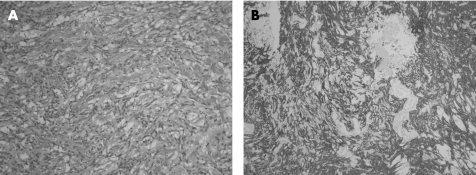
Histologically, all cases shared similar features and showed islands and cords of tumour cells amidst the basophilic myxoid stroma (fig 5). The tumour cells featured spindle to epithelioid appearance, with eosinophilic cytoplasm (fig 6A). The nuclei possessed finely stippled chromatin and discernible nuclear pseudoinclusions. Mitotic activity was not seen. Sheet‐like area was not evident. Immunohistochemically, the tumour cells showed diffuse and strong membranous and cytoplasmic staining for epithelial membrane antigen, strong cytoplasmic staining for vimentin and isolated positive immunostaining for AE1/AE3; stainings for glial fibrillary acidic protein and S‐100 protein were negative (fig 6B). The proliferative index with Mib‐1 was 1–2%.
Figure 5 Frozen section of the tumour showing cords of tumour cells amidst basophilic myxoid stroma (case 3), H&E, ×100.
Figure 6 (A) Paraffin section showing spindle to epithelioid tumour cells with eosinophilic cytoplasm, fine stippled chromatin and discernible intranuclear inclusions. Myxoid material is noted in the background (case 2), H&E, ×100; (B) The tumour cells are strongly immunoreactive towards epithelial membrane antigen (immunoperoxidase).
Discussion
Meningioma is one of the most common primary tumours of the central nervous system. According to the definition by the WHO classification, they are generally slow‐growing tumours that attach to the dura mater, and originate from meningothelial cells that can be found in arachnoid villi, as well as in the arachnoid membrane of the craniospinal space.1 Epidemiologically, they are twice as common in females than in males, with the highest incidence after the fifth decade of life.1 They account for 13–26% of all primary intracranial tumour with an annual incidence rate of approximately 6/100 000 population.2
Histologically, meningiomas are divided into three grades, with about 90% being WHO grade I, and they usually pursue a benign clinical course. Atypical meningiomas (WHO grade II, 5–7% of cases) and anaplastic meningiomas (WHO grade III, 1–3%) are known to behave more aggressively, with recurrence and extensive infiltration, leading to considerable morbidity and mortality.3
According to the current WHO classification, chordoid meningioma is classified into WHO grade II. The term “chordoid meningioma” was first coined by Kepes et al4 in 1988, describing a novel variant of meningeal tumour in young patients. In that series, patients were young, ranging from 6 to 19 years of age, and had systemic involvement of Castleman's disease—namely, iron‐refractory hypochromic/microcytic anaemia—which disappeared on surgical removal of the tumour. They also described that the administration of corticosteroids was associated with complete resolution of systemic manifestations and a significant decrease in the tumour volume. Classically, the tumour is composed of spindle or epithelioid cells forming chordoma‐like clusters and cords in a mucoid matrix, and are associated with prominent lymphoplasmacytic infiltrates. Since then, cases reported in the literature comprised intracranial5,6,7,8,9 and extracranial10 tumours.
In our cases, the patients were middle‐aged adults. The main symptoms were attributed to the mass effect of the tumour. In contrast to the original description by Kepes et al,4 there was no evidence of haematological abnormality or other systemic manifestation. In a large series published by Couce et al11 in 2000, they found that there is no significant link between chordoid meningioma and systemic or haematological abnormality in adults, and suggested that tumoural inflammatory reaction and the presence of haematological manifestation pertain only to paediatric patients, and concluded that chronic inflammatory infiltrate is not a mandatory histopathological criterion for diagnosing chordoid meningioma. Moreover, all the cases showed low proliferation index, implying that the proliferative rate may not be directly related to the common recurrence behaviour of the tumour.
Apart from grading, surgical clearance governs the prognosis of meningioma.12 Chordoid meningioma is particularly prone to recurrence. Couce et al11 demonstrated that all partially resected chordoid meningiomas recurred even after postoperative intervals of up to 16 years. A total of 39% of the patients experienced ⩾1 recurrences between 1.8 and 16 years postoperatively (mean 5.6 years), and in 85.7% of the recurrence‐associated cases the primary tumours were >50% chordoid in pattern and featured little or no inflammatory infiltrate. It was suggested that the mucoid nature of the stroma provides mechanical advantage for tumour spread and thus recurrence. In case 1, the patient had extensive involvement in the right orbit and the right temporalis during recurrence, and this recurrence was predominately chordoid in pattern.
Imprint smear preparation is an indispensable means of intraoperative diagnosis for various brain tumours, with superb diagnostic accuracy. Reyes et al13 demonstrated that 82% of the imprints, 92% of the smears and 99% of the frozen sections correlated with the final diagnosis on paraffin sections. Firlik et al14 found that intraoperative cytological investigation correlated with the final diagnosis in 90% of cases where intraoperative diagnoses were most accurate, in cases of abscess, germinoma, lymphoma, metastasis and malignant glioma. In their study, intraoperative cytological preparations correlated with the final diagnoses in 90% of stereotactic biopsies and had 96% sensitivity in detecting diagnostic specimens. Our group has previously shown that many brain tumour groups have distinct cytological appearances on a smear preparation.15,16,17,18,19
Several studies have shown that chordoid meningioma exhibits distinctive cytological features of cord‐like epithelioid cells, having indistinct cellular border, round, oval nuclei, stippled chromatin and intranuclear inclusions, and that they could be reliably diagnosed by imprint smears.20,21 In our cases, cohesive cords of tumour cells, cytoplasmic vacuolation and background chronic inflammatory cells were the concordant features, being present in all cases in this series. In addition, intranuclear inclusion (4 of 5 cases (80%)) and the presence of whorl‐like structure (3 of 5 cases (60%)) helped to delineate the meningiotheliomatous nature of the tumour cells (table 2). However, none of our cases showed psammoma body, which is a common feature of meningioma.
Table 2 Components assessed from the squash smear.
| Case number | Cellularity | Cellular atypia | Mitotic figure | Cytoplasmic vacuolation | Intranuclear inclusion | Cohesive cords of tumour cells | Whorl‐like structure | Psammoma bodies | Chronic inflammatory cells | Background myxoid material | Necrotic material | Histological section: ratio of percentages of chordoid and meningothelial components |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1 | Moderate | Absent | No | Present | Present | Present | Absent | Absent | Present | Absent | Absent | 90:10 |
| Case 2 | Moderate | Absent | No | Present | Present | Present | Absent | Absent | Present | Absent | Absent | 95:5 |
| Case 3 | High | Absent | No | Present | Absent | Present | Present | Absent | Present | Present | Absent | 80:20 |
| Case 4 | High | Absent | No | Present | Present | Present | Present | Absent | Present | Absent | Absent | 50:50 |
| Case 5 | Moderate | Absent | No | Present | Present | Present | Present | Absent | Present | Present | Absent | 80:20 |
There are many other neoplasms that mimic chordoid meningioma. However, they can be confidently distinguished by careful cytological, histological immunohistochemical assessment.
Chondroid chordoma poses a diagnostic challenge to surgical pathologists, as they may be confused with meningioma in the midline. Cytologically, chordoma may give a similar metachromatic mucofibrillary background on smear, but, in contrast, there are scattered physaliferous cells with irregularly enlarged nuclei and cytoplasmic vacuoles, which are absent in meningioma cells. Histologically, they are usually positive for cytokeratin and S‐100 protein, with only sparse epithelial membrane antigen immunoreactivity.
Chordoid glioma shows similar histology as chordoid meningioma. So far, there is no case report on the cytological findings on chordoid glioma, and a detailed account on the distinction between the two cannot be made. In this setting, the preoperative imaging study definitely helps, as meningioma is usually dura‐based. In addition, paediatric patients with chordoid meningioma often feature a more prominent reactive lymphoid component. Chordoid glioma, however, is not dura‐based and demonstrates no intranuclear inclusion.22 Careful correlation with intraoperative findings usually facilitates differential diagnosis. Histologically, chordoid glioma shows positive immunoreactivity towards glial fibrillary acidic protein, which is negative in meningioma.
Metastatic adenocarcinoma may potentially be confused with chordoid meningioma. The higher degree of anisonucleosis, hyperchromasia, intracytoplasmic mucin vacuoles, formation of three‐dimensional cell balls or papillae in smears, tumour diathesis in the background and a diffuse immunoreactivity towards cytokeratin would favour a diagnosis of the former.
Extraskeletal myxoid chondrosarcoma may be confused with chordoid meningioma. Intracranial extraskeletal myxoid chondrosarcoma in jugular foramen has been reported previously.23 Extracranial locations, such as lung, or as in case 1, with the tumour infiltrating into the extracranial sites, may cause a diagnostic problem. The former characteristically shows cylindrical cells with eccentric nuclei and occasional nuclear grooves,24 and, histologically, displays positive immunostaining for HMB45, synaptophysin, chromogranin and S‐100 protein, facilitating differentiation from chordoid meningioma.
In summary, our cases illustrate the cytological characteristics of chordoid meningioma, a rare neoplasm of the meninges, which is prone to recur after years of apparent remission. Haematological abnormality is not essential for diagnosis in adult patients. The cytological features are quite characteristic, and intraoperative cytological preparation is usually diagnostic.
Take‐home message
The cytological features of chordoid mengiomas are distinctive and intraoperative diagnosis is feasible.
Abbreviations
WHO - World Health Organization
Footnotes
Competing interests: None declared.
References
- 1.Louis D N, Scheithauer B W, Budka H.et al Meningiomas. In: Kleihues P, Cavenee WK, eds. Pathology and genetics of tumours of the nervous system: World Health Organization classification of tumours Lyon: IARC Press, 2000176–184.
- 2.Longstreth W T, Dennis L K, McGuire V M.et al Epidemiology of intracranial meningiomas. Cancer 199372639–648. [DOI] [PubMed] [Google Scholar]
- 3.Perry A, Scheithauer B W, Stafford S L.et al ‘Malignancy' in meningiomas: a clinicopathologic study of 116 patients, with grading implications. Cancer 1999852046–2056. [DOI] [PubMed] [Google Scholar]
- 4.Kepes J J, Chen W Y, Connors M H.et al “Chordoid” meningeal tumors in young individuals with peritumoral lymphoplasmacellular infiltrate causing systemic manifestations of the Castleman syndrome: a report of seven cases. Cancer 198862391–406. [DOI] [PubMed] [Google Scholar]
- 5.Ozen O, Sar A, Atalay B.et al Chordoid meningioma: rare variant of meningioma. Neuropathology 200424243–247. [DOI] [PubMed] [Google Scholar]
- 6.Varma D R, Rao B R, Parameswaran S.et al Chordoid meningioma: a report of two cases. Neurology India 200351522–524. [PubMed] [Google Scholar]
- 7.Yeon J Y, Lee J I, Kim J H.et al Chordoid meningioma: a case report. J Korean Med Sci 200318768–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayadi‐Kaddour A, Kchir N, Bellil K.et al Chordoid meningioma: a case report. Ann Pathol 20032376–78. [PubMed] [Google Scholar]
- 9.de Tella O I, Jr, Herculano M A, Prandini M N.et al Chordoid meningioma: report of two cases. Arq Neuropsiquiatr 20036191–94. [DOI] [PubMed] [Google Scholar]
- 10.Rowsell, Sirbovan J, Rosenblum M K.et al Primary chordoid meningioma of lung. Virchows Archiv 200546333–337. [DOI] [PubMed] [Google Scholar]
- 11.Couce M E, Aker F V, Scheithauer B W. Chordoid meningioma: a clinicopathologic study of 42 cases. Am J Surg Pathol 200024899–905. [DOI] [PubMed] [Google Scholar]
- 12.Jaaskelainen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients. A multivariate analysis. Surg Neurol 198626461–469. [DOI] [PubMed] [Google Scholar]
- 13.Reyes M G, Homsi M F, McDonald L W.et al Imprints, smears, and frozen sections of brain tumors. Neurosurgery 199129575–579. [DOI] [PubMed] [Google Scholar]
- 14.Firlik K S, Martinez A J, Lunsford L D. Use of cytological preparations for the intraoperative diagnosis of stereotactically obtained brain biopsies: a 19‐year experience and survey of neuropathologists. J Neurosurg 199991454–458. [DOI] [PubMed] [Google Scholar]
- 15.Ng H K. Cytologic features of central neurocytomas of the brain. A report of three cases. Acta Cytol 199943252–256. [DOI] [PubMed] [Google Scholar]
- 16.Teo J G, Ng H K. Cytodiagnosis of pilocytic astrocytoma in smear preparations. Acta Cytol 199842614–618. [DOI] [PubMed] [Google Scholar]
- 17.Ng H K. Smears in the diagnosis of pituitary adenomas. Acta Cytol 199842614–618. [DOI] [PubMed] [Google Scholar]
- 18.Ng H K. Cytologic diagnosis of intracranial germinomas in smear preparations. Acta Cytol 199539693–697. [PubMed] [Google Scholar]
- 19.Ng H K. Cytologic features of ependymomas in smear preparations. Acta Cytol 199438331–334. [PubMed] [Google Scholar]
- 20.Inagawa H, Ishizawa K, Shimada S.et al Cytologic features of chordoid meningioma. A case report. Acta Cytol 200448397–401. [DOI] [PubMed] [Google Scholar]
- 21.Salinero E, Beltran L, Costa J R. Intraoperative cytologic diagnosis of chordoid meningioma. A case report. Acta Cytol 200448259–263. [DOI] [PubMed] [Google Scholar]
- 22.Pasquier B, Peoc'h M, Morrison A L.et al Chordoid glioma of the third ventricle: a report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. Am J Surg Pathol 2002261330–1342. [DOI] [PubMed] [Google Scholar]
- 23.Cummings T J, Bridge J A, Fukushima T. Extraskeletal myxoid chondrosarcoma of the jugular foramen. Clin Neuropathol 200423232–237. [PubMed] [Google Scholar]
- 24.Domanski H A, Carlen B, Mertens F.et al Extraskeletal myxoid chondrosarcoma with neuroendocrine differentiation: a case report with fine‐needle aspiration biopsy, histopathology, electron microscopy, and cytogenetics. Ultrastruct Pathol 200327363–368. [DOI] [PubMed] [Google Scholar]