Involvement of the parathyroid glands by metastatic tumour is rare. In autopsy studies of known cancer patients, it was noted in 0.2–11.9% of individuals.1 Hypoparathyroidism and hypocalcaemia as a result of parathyroid destruction by tumour is unusual.2,3 We report a case of hyperparathyroidism due to parathyroid hyperplasia with simultaneous occurrence of metastatic bronchogenic adenocarcinoma to a parathyroid gland.
Case report
A 75‐year‐old woman was referred with hypercalcaemia. Six months earlier she had presented to an osteoporosis clinic with generalised pain in the upper limbs. She reported anorexia and mild weight loss but was otherwise asymptomatic. Specifically there were no respiratory symptoms. A bone density scan revealed osteoporosis. Routine biochemical investigations revealed hypercalcaemia, raised parathyroid hormone level and normal renal function (table 1). A parathyroid pertechnetate/MIBI subtraction scan suggested the presence of an enlarged left superior parathyroid gland. The patient was a non‐smoker and had no significant past medical history. Plain radiographs of the chest and renal tracts taken 6 months prior to surgery were normal. A diagnosis of primary hyperparathyroidism seemed secure and surgical exploration advised. Prior to operation a hard palpable lymph node in the right submandibular region was noted and it was planned to excise this at the same time as neck exploration.
Table 1 Blood biochemistry results.
| At presentation 6 months before surgery | Preoperative 5 months before surgery | Postoperative Day 2 post‐surgery | Normal range | |
|---|---|---|---|---|
| Calcium (mmol/l) | 2.73 | 2.77 | 2.16 | 2.22–2.66 |
| Albumin (g/l) | 35 | 34 | 30 | 35–50 |
| Corrected calcium (mmol/l) | 2.83 | 2.89 | ND | |
| Phosphate (mmol/l) | 0.70 | 0.88 | 1.03 | 0.80–1.55 |
| Alkaline phosphatase (U/l) | 109 | 123 | ND | 35–120 |
| Parathyroid hormone (pg/ml) | 181 | 191 | ND | 10–85 |
| Urea (mmol/l) | 6.7 | 5.2 | 3.3–8.8 | |
| Creatinine (µmol/l) | 51 | 55 | 40–110 |
ND, not done.
A unilateral left sided neck exploration was carried out using the surgical strategy which we have previously described.4 At operation, an enlarged left superior parathyroid gland was identified and removed. A normal sized left inferior parathyroid gland was excised for comparative biopsy and the right submandibular node resected. Intraoperative pathological examination was not done. The right side of the neck was not explored. The postoperative period was uneventful and the serum calcium returned to normal. On receipt of the histopathological report the patient was readmitted for further investigations. Ear, nose and throat examination showed no abnormality. Computed tomography of the neck and chest now showed marked mediastinal lymphadenopathy and left lower lung consolidation associated with a pleural effusion. A subsequent FDG‐PET (fluorodeoxyglucose positron emission tomography) scan revealed abnormal FDG uptake in the right occipital lobe of the brain, in mediastinal, pulmonary hilar, paraaortic and supraclavicular lymph nodes, in the right adrenal gland and both iliac bones, and in lumbar (L2) and thoracic (T5) vertebral bodies. The findings were consistent with widespread metastatic disease from a presumed primary bronchial carcinoma. The patient was unfit for any further diagnostic procedure. She underwent a course of palliative radiotherapy but died a few months later. Autopsy was not performed.
Pathological findings
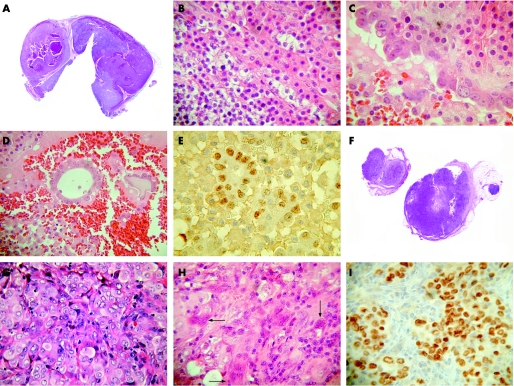
The left superior parathyroid gland measured 33 mm in maximum dimension and weighed 4173 mg. The cut surface was nodular and brown in colour. The left inferior parathyroid measured 5 mm in maximum dimension and weighed 120 mg. Microscopy showed nodular hyperplasia of chief cells and oxyphil cells in both glands. Areas of haemorrhage and cystic degeneration were present in the larger left superior gland. Hobnail cells with vesicular nuclei and prominent nucleoli lined one of these cysts. The cyst lumen contained a few malignant glands. A diagnosis was made of parathyroid hyperplasia of both glands with metastatic adenocarcinoma within the left superior parathyroid. The right submandibular lymph node measured 12 mm in diameter and was totally replaced by metastatic poorly differentiated carcinoma with a stroma rich in osteoclastic giant cells. The tumour cells had vesicular nuclei and prominent nucleoli similar to that of the metastatic carcinoma in the left parathyroid gland but neither glandular nor squamous differentiation was evident. Immunohistochemical staining showed the tumour cells staining positively with CK7, PE10 and TTF‐1, within both the lymph node and the left superior parathyroid gland. There was no reactivity to WT‐1, CA‐125, CK20, and HMB‐45. The immunoprofile suggested a diagnosis of primary bronchogenic carcinoma with metastasis to the right submandibular lymph node and the left superior parathyroid gland (fig 1).
Figure 1 (A) Nodular hyperplasia of the left superior parathyroid gland with areas of haemorrhage and cystic degeneration. (B) Chief cells and oxyphil cells in the hyperplastic left inferior parathyroid gland. (C) Left superior parathyroid gland cyst lined by malignant cells contrasting with bland chief/oxyphil cells in the wall of the cyst. (D) Malignant glands within the left superior parathyroid gland cyst. (E) Malignant glands within left superior parathyroid cyst showing TTF‐1 nuclear staining. (F) Section of lymph node replaced by metastatic carcinoma. (G) Sheet of carcinoma cells within the lymph node with occasional gland‐like spaces. (H) Metastatic carcinoma with osteoclastic giant cells (arrows) within lymph node. (I) Metastatic carcinoma within lymph node showing TTF‐1 nuclear staining.
Discussion
We have reported a patient with primary hyperparathyroidism due to parathyroid hyperplasia and coincidental metastatic adenocarcinoma involving one of the enlarged parathyroid glands. While there are independent reports of metastatic tumour involving the parathyroids and primary hyperparathyroidism associated with disseminated malignancy, a literature search revealed no reported case similar to ours wherein the metastatic adenocarcinoma was identified as a result of parathyroid gland excision for hyperparathyroidism.
Metastasis of tumour to the parathyroid glands has been described previously in autopsy studies of known cancer patients with widespread tumour.1,2 The commonest sites of primary tumour in these circumstances were breast, lung, and soft tissue, also leukaemia and cutaneous melanoma.3 Secondary involvement of the parathyroid gland by local invasion of thyroid and laryngeal carcinomas is also reportedly infrequent.5 Tang et al have described involvement of the parathyroid gland by papillary thyroid carcinoma in 20 of 911 cases; 2% of these had metastases as opposed to direct invasion.6 Our case therefore appears to be unique in that the patient presented with symptoms related to hypercalcaemia and the primary lung tumour was undetected prior to removal of the parathyroid glands.
The association of hypercalcaemia with non‐parathyroid cancer is well recognised. The mechanisms of the hypercalcaemia include osteolytic metastasis and hypercalcaemia developing as a non‐metastatic phenomenon consequent to production of a parathyroid hormone‐like humoral agent by the tumour.5,7,8 There are several case reports of hypercalcaemia due to primary hyperparathyroidism associated with cancer of the lung, and non‐medullary carcinoma of the thyroid, breast and larynx, but the frequency of this association is not precisely known. Many of these cancer patients had hypercalcaemia in the absence of metastatic disease; the presence of raised parathyroid hormone levels led to evaluation of the parathyroid glands.9,10,11,12,13 Godsall et al noted primary hyperparathyroidism with concomitant non‐parathyroid cancer in 8 of 133 patients with disseminated cancer. The types of cancer included squamous lesions of the head and neck, lung, and colon, breast adenocarcinoma and myeloma.14 Honda et al reported primary hyperparathyroidism occurring in association with aldosterone‐producing adrenocortical adenoma and breast cancer, and suggested relation to MEN1 gene mutations.15 However, there are others who suggest that the occurrence of the two diseases is coincidental rather than due to a shared aetiology.13 Primary hyperparathyroidism due to parathyroid adenoma has also been reported with a slightly increased frequency in non‐aggressive breast cancer patients.9,10 In our patient, despite the gross impression of a parathyroid adenoma, microscopy of the left superior and inferior parathyroid glands showed hyperplasia with asymmetric enlargement of the left superior parathyroid gland. The serum calcium reverted to normal after parathyroid exploration. It seems probable that removal of the larger parathyroid gland was responsible for normalisation of the serum calcium and that the microscopic hyperplasia of the second parathyroid gland was functionally insignificant.
In conclusion, this report describes the rare incidental discovery of metastatic adenocarcinoma within a hyperplastic parathyroid gland resected for surgical management of primary hyperparathyroidism. It is important for clinicians and pathologists to be aware of this possibility in patients evaluated for osteoporosis and hypercalcaemia.
Acknowledgements
We thank Dr Seamus Napier and Mr Craig McLaughlin for assistance with taking the photographs.
Footnotes
Competing interests: None.
References
- 1.Gattuso P, Khan N A, Jablokow V R.et al Neoplasms metastatic to parathyroid glands. South Med J 1988811467. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz C A, Myers W P, Foote F W., Jr Secondary malignant tumors of the parathyroid gland. Report of 2 cases with associated hypoparathyroidism. Am J Med 197252797–808. [DOI] [PubMed] [Google Scholar]
- 3.De la Monte S M, Hutchins G M, Moore G W. Endocrine organ metastases from breast carcinoma. Am J Pathol 1984114131–136. [PMC free article] [PubMed] [Google Scholar]
- 4.Sidhu S, Neill A K, Russell C F J. Long‐term outcome of unilateral parathyroid exploration for primary hyperparathyroidism due to presumed solitary adenoma. World J Surg 200327339–342. [DOI] [PubMed] [Google Scholar]
- 5.DeLellis R A. Miscellaneous lesions. In: DeLellis RA, ed. Atlas of tumor pathology. Tumors of the parathyroid gland Washington, DC: Armed Forces Institute of Pathology, 199393–94.
- 6.Tang W, Kakudo K, Nakamura Y.et al Parathyroid gland involvement by papillary carcinoma of the thyroid gland. Arch Pathol Lab Med 2002261511–1514. [DOI] [PubMed] [Google Scholar]
- 7.Stewart A F. Clinical practice. Hypercalcemia associated with cancer. N Engl J Med 2005352373–379. [DOI] [PubMed] [Google Scholar]
- 8.Solimando D A. Overview of hypercalcemia of malignancy. Am J Health Syst Pharm 200158(Suppl 3)4–7. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto J, Kojima T, Shimizu T.et al A case of lung cancer with hypercalcemia which was incidentally complicated with primary hyperparathyroidism due to parathyroid adenoma. Ann Thorac Cardiovasc Surg 20023151–153. [PubMed] [Google Scholar]
- 10.Attie J N, Vardhan R. Association of hyperparathyroidism with nonmedullary thyroid carcinoma: review of 31 cases. Head Neck 1993120–23. [DOI] [PubMed] [Google Scholar]
- 11.Kara I O, Sahin B, Yapar Z. Breast cancer and concomitant primary hyperparathyroidism: description of two patients. Acta Med Austriaca 2004381–84. [PubMed] [Google Scholar]
- 12.Axelrod D M, Bockman R S, Wong G Y.et al Distinguishing features of primary hyperparathyroidism in patients with breast cancer. Cancer 1987601620–1624. [DOI] [PubMed] [Google Scholar]
- 13.Haar J G, Boulos E J. Primary hyperparathyroidism and laryngeal carcinoma: a cause of associated hypercalcemia. Laryngoscope 1981111937–1940. [DOI] [PubMed] [Google Scholar]
- 14.Godsall J W, Burtis W J, Insogna K L.et al Nephrogenous cyclic AMP, adenylate cyclase stimulating activity and the humoral hypercalcemia of malignancy. Recent Prog Horm Res 198642705–750. [DOI] [PubMed] [Google Scholar]
- 15.Honda M, Tsukada T, Horiuchi T.et al Primary hyperparathyroidism associated with aldosterone‐producing adrenocortical adenoma and breast cancer: relation to MEN1 gene. Intern Med 200443310–314. [DOI] [PubMed] [Google Scholar]