HER2 protein overexpression or gene amplification is detected in approximately 20% of breast tumours, is associated with an aggressive phenotype and is predictive of response to trastuzumab therapy. The use of chromogenic in situ hybridisation (CISH) for evaluating HER2 status routinely is recognised in much of Europe but has yet to achieve widespread acceptance in the UK. CISH analysis for HER2 status was performed on 161 breast cancer cases. Results were compared with immunohistochemistry (IHC) and dual‐colour fluorescence in situ hybridisation (FISH) data. There was 100% concordance between CISH and FISH, but only 93.8% concordance between CISH and IHC. Performing CISH ‘in‐house' was found to cost approximately 50% less than the FISH/IHC protocol at the reference laboratory. It is concluded that CISH is as accurate as FISH for diagnostic purposes and is more cost‐effective than the IHC/FISH regimen currently favoured in the UK.1
Recent data show that amplification of the HER‐2 gene is the most reliable predictor of response to trastuzumab therapy,2 indicating that a gene‐based assay, rather than a protein overexpression assay, would be the most suitable type of analysis for HER2 status in breast tumour samples.
With the recent change in the licensing of trastuzumab to include its use as an adjuvant therapy (NICE 2006), it is important that patients most likely to benefit from its use are accurately identified. This change in the use of trastuzumab has increased the workload of histopathology laboratories significantly as well as creating an additional financial burden for hospital Trusts. We investigated the possibility of setting up HER2 analysis within our pathology department. Chromogenic in situ hybridisation (CISH), like fluorescence in situ hybridisation (FISH), directly visualises the number of gene copies present in the nucleus, it is cheaper and it produces a permanent record of the slide that can be interpreted with a light microscope in the context of the tumour histopathology. CISH and FISH have been compared for their sensitivity and specificity in numerous previous reports from across Europe,3,4,5,6,7 but CISH is not widely used in the UK.1 The current study was done as a validation study prior to setting up a HER2 testing service using CISH, but it was felt that our experience may be of interest to other laboratories considering setting up their own HER2 testing service.
Methods
One hundred and sixty‐one breast cancer cases for which material was obtainable and that had immunohistochemistry (IHC) and/or FISH data available were chosen for the study. The samples had been analysed by the DAKO HercepTest IHC (DakoCytomation, Ely, Cambridgeshire, UK) and a small number of them (n = 24) had required confirmation by PathVysion dual‐colour FISH (Abbott UK, Queensborough, Kent, UK) mostly because they were IHC 2+. The manufacturer's protocols and scoring systems for both procedures were followed. The same cases were subsequently examined using a commercially available CISH assay (Zymed, Invitrogen, Paisley, UK), and the manufacturer's protocol was followed. The resulting slides were examined independently by two pathologists using light microscopy. The areas of invasive tumour were identified and the HER2 status was scored using the manufacturer's guidelines. Amplification was recorded when the nuclei of >50% cells contained clusters, multiple dots (>5) or a mixture of both. No amplification was recorded when the nuclei of >50% cells contained one or two small dots. At least four areas of the tumour were examined to overcome issues of heterogeneity. Samples with 3–5 and 5–10 dots were further analysed using a chromosome 17 centromeric probe to confirm highly proliferating tumours in the former and cases of chromosome 17 numerical aberration in the latter. The cost implications for both the protocols were assessed.
Results
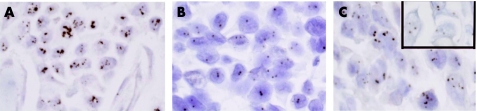
Results were obtained for all 161 samples tested. Amplified and non‐amplified cases were readily distinguishable in the majority of cases (fig 1), whilst chromosome 17 correction was required in 19 cases (10.6%) to confirm interpretation.
Figure 1 (A) Core biopsy of invasive ductal carcinoma. Cells have been hybridised with a HER2 gene probe and visualised using an anti‐digoxigenin peroxidase antibody, developed with 3',3'‐diaminobenzidine. Chromogenic in situ hybridisation analysis shows large clusters of hybridised HER2 probe, indicating the presence of a high level of HER2 gene amplification. (B) Core biopsy of invasive ductal carcinoma. One or two dots can be seen in each nucleus, indicating the absence of HER2 gene amplification. (C) Core biopsy of invasive ductal carcinoma. Small clusters and multiple dots can be seen, indicating a low level amplification of the HER2 gene. The insert shows analysis using the probe for the chromosome 17 centromere which indicates absence of polysosmy.
CISH and IHC showed 93.8% concordance (151/161) (table 1). Dual‐colour FISH and CISH showed 100% concordance (table 1). There were seven (4.4%) IHC 3+ cases that were found by CISH to be not amplified. In each one of these seven cases, the results obtained with CISH were re‐confirmed by dual‐colour FISH analysis. One case (0.6%), originally scored IHC 1+, showed amplification by CISH, again re‐confirmed by dual‐colour FISH. There was 100% agreement between the two examining pathologists.
Table 1 HER2 1HC and FISH versus CISH data.
| IHC | FISH | |||||||
|---|---|---|---|---|---|---|---|---|
| 3+ | 2+ | 0/1+ | + | − | ||||
| CISH | + | 29 | 4 | 1 | CISH | + | 5 | 0 |
| − | 7 | 12 | 108 | − | 0 | 19 | ||
CISH, chromogenic in situ hybridisation; FISH, fluorescence in situ hybridisation; IHC, immunohistochemistry;
Discussion
FDA (Food and Drug Administration) approved HercepTest IHC analysis has been adopted as the frontline test for identifying patients eligible for trastuzumab therapy. A total of 5% of the samples in our series did not show the expected correspondence between HER2 gene status and HER2 protein expression, similar to previous reports.4,8 Our data indicate that 11% of women eligible for trastuzumab therapy on the basis of the IHC result are unlikely to benefit from it.
Take‐home messages
Use of an in situ hybridisation assay in place of immunohistochemsitry would identify more accurately those women who would benefit from trastuzumab therapy.
Chromogenic in situ hybridisation (CISH) is as sensitive and as specific as fluorescence in situ hybridisation (FISH)
CISH is more cost‐effective than FISH
Misdirected therapy will potentially cost health authorities significant sums of money. A recent cost‐effectiveness analysis for HER2 testing and trastuzumab therapy9 concluded that it is more cost‐effective to use FISH alone or as confirmation of all IHC 2+ and 3+ results, rather than the current system. Whilst an expanded two‐tier analysis will address the issue of false positivity, it will not identify those IHC‐negative/FISH‐positive cases that we and others have found in a proportion of tumours (0.6–4.4%).10 Therefore, a frontline ISH test would be a more favourable option. Dual‐colour FISH is accepted as the gold standard for HER2 analysis; CISH has been shown here and previously5,11,12,13 to be as sensitive and specific. Other reports have shown a high but variable level of concordance (93.8–96%).3,6,14 The small number of discordant results in these reports have frequently been in the borderline samples due to the slight difference in cut‐off level between CISH and FISH.14 A HER2:CEP17 ratio of >2 is classed as amplified by FISH; however, with CISH (Zymed), a score of >5 HER2 signals is classed as amplified. A consensus should be reached on a clinically relevant cut‐off value for amplification for both ISH protocols.
Other FISH/CISH discrepancies have been due to inadequate use of chromosome 17 correction and difficult histology.3,6 By using chromosome 17 correction in all low amplified cases, light microscopy for assessment of HER2 in the histological context of the tumour sample and having experienced histopathologists analysing all the slides, we have not encountered these discrepancies between FISH and CISH results leading us to believe that CISH can reliably be used to assess breast tumour samples for patient eligibility for trastuzumab therapy.
Acknowledgements
We would like to thank Roche Products Ltd for the funding received for this study. The authors are independent of the funding organisation which sought no control over the design or content of the study.
Footnotes
Competing interests: none declared.
References
- 1.Ellis I O, Bartlett J, Dowsett M.et al Best Practice No 176: Updated recommendations for HER2 testing in the UK. J Clin Pathol 200457233–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon D J, Leyland‐Jones B, Shak S.et al Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001344783–792. [DOI] [PubMed] [Google Scholar]
- 3.Saez A, Andreu F A, Segui M A.et al HER‐2 gene amplification by chromogenic in situ hybridisation (CISH) compared with fluorescence in situ hybridisation (FISH) in breast cancer—a study of two hundred cases. Breast 200615519–527. [DOI] [PubMed] [Google Scholar]
- 4.Pauletti G, Dandekar S, Rong H.et al Assessment of methods for tissue‐based detection of the HER‐2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol 2000183651–3664. [DOI] [PubMed] [Google Scholar]
- 5.Gong Y, Gilcrease M, Sneige N. Reliability of chromogenic in situ hybridization for detecting HER‐2 gene status in breast cancer: comparison with fluorescence in situ hybridization and assessment of interobserver reproducibility. Mod Pathol 2005181015–1021. [DOI] [PubMed] [Google Scholar]
- 6.Isola J, Tanner M, Forsythe A.et al Interlaboratory comparison of HER‐2 oncogene amplification as detected by chromogenic and fluorescence in situ hybridization. Clin Cancer Res 2004104793–4798. [DOI] [PubMed] [Google Scholar]
- 7.Laakso M, Tanner M, Isola J. Dual‐colour chromogenic in situ hybridization for testing of HER‐2 oncogene amplification in archival breast tumours. J Pathol 20062103–9. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett J M, Going J J, Mallon E A.et al Evaluating HER2 amplification and overexpression in breast cancer. J Pathol 2001195422–428. [DOI] [PubMed] [Google Scholar]
- 9.Elkin E B, Weinstein M C, Winer E P.et al HER‐2 testing and trastuzumab therapy for metastatic breast cancer: a cost‐effectiveness analysis. J Clin Oncol 200422854–863. [DOI] [PubMed] [Google Scholar]
- 10.Sauer T, Wiedswang G, Boudjema G.et al Assessment of HER‐2/neu overexpression and/or gene amplification in breast carcinomas: should in situ hybridization be the method of choice? Apmis 2003111444–450. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava R, Lal P, Chen B. Chromogenic in situ hybridization for the detection of HER‐2/neu gene amplification in breast cancer with an emphasis on tumors with borderline and low‐level amplification: does it measure up to fluorescence in situ hybridization? Am J Clin Pathol 2005123237–243. [PubMed] [Google Scholar]
- 12.Hauser‐Kronberger C, Dandachi N. Comparison of chromogenic in situ hybridization with other methodologies for HER2 status assessment in breast cancer. J Mol Histol 200435647–653. [DOI] [PubMed] [Google Scholar]
- 13.Dandachi N, Dietze O, Hauser‐Kronberger C. Chromogenic in situ hybridization: a novel approach to a practical and sensitive method for the detection of HER2 oncogene in archival human breast carcinoma. Lab Invest 2002821007–1014. [DOI] [PubMed] [Google Scholar]
- 14.Hanna W M, Kwok K. Chromogenic in‐situ hybridization: a viable alternative to fluorescence in‐situ hybridization in the HER2 testing algorithm. Mod Pathol 200619481–487. [DOI] [PubMed] [Google Scholar]