Abstract
Background
Cutaneous lymphomas expressing CD56, a neural cell adhesion molecule, are characterised in most cases by a highly aggressive clinical course and a poor prognosis. However, prognostic subsets within the CD56+ group have been difficult to identify due to the lack of uniform clinicopathological and immunophenotypical criteria.
Methods
A multicentre study was conducted by the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer to define prognostic parameters and establish diagnostic and therapeutic guidelines for CD56+ haematological neoplasms presenting primarily in the skin.
Results
Four different subtypes of lymphoproliferations with CD56 expression were identified: (1) haematodermic neoplasm; (2) skin infiltration as the first manifestation of CD56+ acute myeloid leukaemia; (3) nasal‐type extranodal natural killer/T‐cell lymphoma; and (4) “classical” cases of cutaneous T‐cell lymphoma (CTCL) with co‐expression of the CD56 molecule. Patients in the first three groups had a poor outcome (93% died) with a median survival rate of 11 months (95% CI 2–72 months), whereas all patients with CD56+ CTCL were alive at the last follow‐up.
Conclusion
Results show that CD56+ cutaneous lymphoproliferative disorders, with the exception of CD56+ CTCL have a very poor prognosis. It is therefore clinically important to separate CD56+ CTCL from the remaining CD56+ haematological disorders.
Keywords: cutaneous lymphoma, CD56, extranodal NK/T‐cell lymphoma, haematodermic neoplasm, CD123
Cutaneous T‐cell lymphomas are a heterogeneous group of malignancies with mycosis fungoides and Sézary syndrome as the common subtypes. Application of monoclonal antibodies against the neural cell adhesion molecule CD56 has led to the recognition of further lymphoma subtypes.1,2,3 CD56 was initially identified as a surface molecule of CD16+ natural killer (NK) cells with the morphology of large granular lymphocytes. CD56 is expressed not only on all NK cells but also on subsets of CD4+ and CD8+ T cells.4 The WHO–EORTC (World Health Organization–European Organisation for Research and Treatment of Cancer) classification distinguishes between several types of CD56+ neoplasms, including nasal/nasal‐type extranodal NK/T‐cell lymphoma and haematodermic neoplasm (blastic NK‐cell lymphoma).5
Extranodal NK/T cell lymphoma is a tumour occurring in the nasopharyngeal area and frequently shows an angiocentric growth pattern with prominent necrosis leading to the highly characteristic midline perforation once referred to as “lethal midline granuloma”.6 Most cases are positive for CD56 and Epstein‐Barr virus (EBV), and the tumour cells often express cytotoxic molecules such as perforin, granzyme B and TIA‐1.
Haematodermic neoplasm is a recently recognised entity characterised by blastoid tumour cells expressing CD4 and CD56; it has a high incidence of skin involvement and harbours the risk of leukaemic dissemination. Although this tumour has been regarded as an NK‐cell neoplasm, recent studies suggest that it derives from plasmacytoid dendritic cells (pDC).7,8 Both CD56+ malignancies—extranodal NK/T cell lymphoma and haematodermic neoplasm—are associated with a highly aggressive clinical course, and thus a poor prognosis. Although systemic chemotherapy is the first choice for both diseases, the results are often disappointing.9,10
However, occasionally CD56 may be also expressed by other types of disease with involvement of the skin, such as primary cutaneous CD30+ lymphoproliferations, including lymphomatoid papulosis and anaplastic large cell lymphoma, subcutaneous panniculitis‐like T‐cell lymphoma, or myeloid leukaemia with cutaneous infiltration.11,12 Physicians face a challenge in diagnosing and treating this group of well‐defined types of lymphomas/leukaemia expressing the CD56 molecule. It is uncertain whether these patients have a worse prognosis than patients with extranodal NK/T‐cell lymphoma (nasal/nasal type) and haematodermic neoplasm/blastic NK cell lymphoma, and therefore might require a more aggressive therapeutic approach.12
To unravel possible clinical differences among the CD56+ cutaneous disorders we conducted a multicentre study to comparatively analyse the clinical features and histomorphology of this group of CD56+ skin lesions. The aim of this study was to define prognostic parameters and guidelines for the diagnosis and treatment of CD56+ haematological neoplasms first presenting in the skin.
Materials and methods
Patients
A total of 34 cases involving European patients with CD56+ lymphoid tumours presenting primarily in the skin were provided by the members of the EORTC Cutaneous Lymphoma Task Force. The following clinical data were recorded: sex, age at first diagnosis, site of manifestation, clinical presentation, time‐point of cutaneous involvement, involvement of other organs, concurrent existence of other lymphoid or haematopoietic neoplasias, applied therapy, and outcome.
Pathological studies
All biopsy specimens were routinely processed and embedded in paraffin. Tissue sections (5 μm) were cut and stained with H&E, and for immunohistochemistry using a streptavidin–biotin method using antibodies listed in table 1. For antibodies CD33 and CD123, acetone‐fixed frozen tissue sections were stained using the alkaline phosphatase–anti‐alkaline phosphatase technique, as previously described.13
Table 1 Antibodies used for immunophenotyping.
| Marker | Clones used for staining | Manufacturer |
|---|---|---|
| CD3 | Dako | |
| CD3e | PS1 | Novocastra |
| CD4 | 1F6 | Novocastra |
| CD45RO | OPD4 | Dako |
| CD8 | C8/144/3 | Dako |
| CD33 | WM‐54 | Dako |
| CD56 | 123/C3 | Zymed |
| CD57 | F45 | Quartett |
| CD43 | DF‐T1 | Dako |
| CD34 | QBEND10 | Immunotech |
| MPO | A0398 | Dako |
| CD68 | KP1 | Dako |
| TIA | TIA‐1 | Coulter |
| GranB | Gr‐B7 | Coulter |
| CD30 | BerH2 | Dako |
| CD123 | 6H6 | eBioscience |
| EBV‐LMP‐1 | CS1‐4 | Dako |
| EBV‐EBNA‐2 | PE2 | Dako |
| TCL‐1 | 27D6/20 | Biosearch |
| TdT | A3527 | Dako |
Cases were reviewed and classified microscopically by each dermatopathologist/pathologist; consensus criteria were established for the diagnosis of questionable cases. Well‐established entities such as haematodermic neoplasm/blastic NK cell lymphoma and extranodal NK/T cell lymphoma were defined more strictly and cases with unusual morphology or antigen expression could be identified.
TCR‐gamma gene rearrangement studies
DNA used for PCR was extracted from 25 μm paraffin sections after dewaxing and proteinase K digestion using a QIAGEN DNA extraction kit (Qiagen, Hilden, Germany). PCR analysis of T cell receptor gamma (TCR‐gamma) chain rearrangements was performed at the participating institutions as previously described.14,15
Statistical analysis
Survival time was calculated from the on‐study date until the date of lymphoma‐related death or the last follow‐up. The Kaplan–Meier method was used to calculate survival probability as a function of time. The log‐rank test detected significant differences between the survival curves. All data were analysed using SPSS.16 The cut‐off level of statistical significance was set at 0.05 in all analyses.
Results
Definition of groups
Based on the collected clinical, histological, immunohistological, and molecular diagnostic data, four different groups were defined. The majority of cases could be classified in accordance with the WHO and WHO–EORTC classifications—as CD4+/CD56+ haematodermic neoplasm/blastic NK cell lymphoma (group 1) and skin infiltration of CD56+ AML (group 2). In addition, we identified two further subgroups of CD56+ cutaneous disorders, namely, extranodal NK/T cell lymphoma, nasal type (group 3) and CD56+ “classical” primary cutaneous T cell lymphomas (group 4) (tables 2 and 3).5,17
Table 2 Clinical characteristics of patients.
| Characteristics | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Number | 20 | 4 | 5 | 5 |
| Sex | ||||
| Male | 15 (75%) | 3 (75%) | 3 (60%) | 3 (60%) |
| Female | 5 (25%) | 1 (25%) | 2 (40%) | 2 (40%) |
| Age (y) | ||||
| Mean | 65 | 62 | 72 | 54.4.0 |
| Range | 8–86 | 48–92 | 58–73 | 7–84 |
| Extent of skin lesion | ||||
| Solitary | 3 (15%) | 3 (75%) | 2 (40%) | 2 (40%) |
| Generalised | 17 (85%) | 1 (25%) | 3 (60%) | 3 (60%) |
| Involved extracutaneous sites | ||||
| No extracutaneous involvement | 0 (0%) | 0 (100%) | 1 (20%) | 5 (100%) |
| Lymph nodes | 13 (65%) | 1 (25%) | 2 (40%) | 0 (0%) |
| Visceral organs | 0 (0%) | 0 (0%) | 1 (20%) | 0 (0%) |
| Peripheral blood | 4 (20%) | 1 (25%) | 0 (0%) | 0 (0%) |
| Bone marrow | 13 (65%) | 2 (50%) | 1 (20%) | 0 (0%) |
| Palate | 3 (15%) | 0 (0%) | 2 (40%) | 0 (0%) |
| Other neoplasia | 2 (10%)* | 0 (0%) | 0 (0%) | 0 (0%) |
| Therapy | 0 (0%) | |||
| Phototherapy | 0 (0%) | 0 (0%) | 0 (0%) | 1 (20%) |
| Multiagent chemotherapy | 15 (75%) | 3 (75%) | 4 (80%) | 1 (20%) |
| Radiotherapy | 2 (10%) | 1 (25%) | 1 (20%) | |
| Bone marrow transplantation | 2 (10%) | 1 (25%) | 0 (0%) | 0 (0%) |
| Survival, months | ||||
| Median | 12 | 11 | 7 | +62 |
| Range | 3–55 | 3–60 | 2–72 | |
| Current status | ||||
| No evidence of disease | 1 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Alive with disease | 0 (0%) | 0 (0%) | 1 (20%) | 5 (100%) |
| Died of lymphoma | 19 (95%) | 4 (100%) | 4 (80%) | 0 (0%) |
| Died of other cause | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Group 1, haematodermic neoplasm; group 2, cutaneous infiltrates of acute myeloid leukaemia; group 3, extranodal NK/T‐cell lymphoma; group 4, “classical CTCL” with expression of CD56.
*One patient developed in addition a chronic myeloid leukaemia, and another patient a myelodysplastic syndrome.
Table 3 Immunophenotypic and genotypic features of CD56+ lymphoproliferations.
| Antigen | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| CD3 | 0/18 (0%) | 0/4 (0%) | 3/5 (%) | 4/5 (80%) |
| CD3ε | 2/12 (%) | 0/2 (0%) | 2/3 (%) | 1/2 (50%) |
| CD4 | 20/20 (100%) | 4/4 (100%) | 3/5 (%) | 0/5/(0%) |
| CD8 | 0/20 (0%) | 0/3 (0%) | 1/5 (%) | 4/5 (80%) |
| CD30 | 0/20 (0%) | 0/4 (0%) | 0/5 (0%) | 2/5 (40%) |
| CD33, MPO, CD68 | 0/20 (0%) | 4/4 (100%) | 0/2 (0%) | 0/2 (0%) |
| CD43 | 17/19 (90%) | 4/4 (100%) | 1/2 (0%) | 0/2 (0%) |
| CD123 | 6/6 (100%) | 1/2 (50%) | 0/1 (0%) | 0/2 (0%) |
| TCL1 | 4/4 (100%) | 2/3 (67%) | nd | 0/2 (0%) |
| TIA | 0/20 (0%) | 0/3 (0%) | 5/5 (100%) | 4/5(80%) |
| GrB | 0/5 (0%) | 0/2 (0%) | 5/5 (100%) | 4/5 (80%) |
| EBV | 0/13 (0%) | 0/2 (0%) | 3/4 (75%) | 0/4 (0%) |
| TdT | 5/13 (39%) | 0/3 (0%) | 0/1 (0%) | 0/2 (0%) |
| Clonal TCR rearrangement | 0/16 (0%) | 0/3 (0%) | 1/4 (25%) | 3/4 (75%) |
Group 1, haematodermic neoplasm; group 2, cutaneous infiltrates of acute myeloid leukaemia; group 3, extranodal NK/T‐cell lymphoma; group 4, “classical CTCL” with expression of CD56.
MPO, myeloperoxidase; GrB, granzym B; TdT, terminale desoxynucleotidyl transferase.
Group 1: CD4+/CD56+ haematodermic neoplasm (blastic NK‐cell lymphoma)
Clinical features
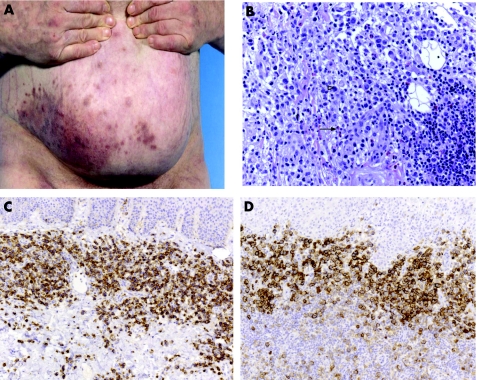
The group comprised 15 men and 5 women (mean age 65 years, range 8–86 years). In most cases, patients presented with multiple red to violet‐brown plaques and nodules which occurred mainly in the trunk, head or leg (9/20 patients) or were generalised (8/20 patients) (fig 1A). Three patients had solitary tumours at different localisations, one exhibiting small satellite nodules (fig 1B). In 15/20 cases the tumours presented in the skin, but subsequent systemic disease developed after a short period. Extracutaneous sites, histologically confirmed at biopsy, included bone marrow (13/20), lymph nodes (8/20), blood (4/20), and palate (2/20). Two patients were reported to have concurrent lymphoid/haematopoietic neoplasias (myelodysplastic syndrome and chronic myeloid leukaemia). Despite various chemotherapy regimens, most patients with available follow‐up (19/20) died within a few months after diagnosis (median survival 12 months, range 3–55 months, 95% CI 6 to 18 months). Notably, one of the two patients who received allogenic bone marrow transplantation had the longest survival time of 60 months, and the other is still alive.
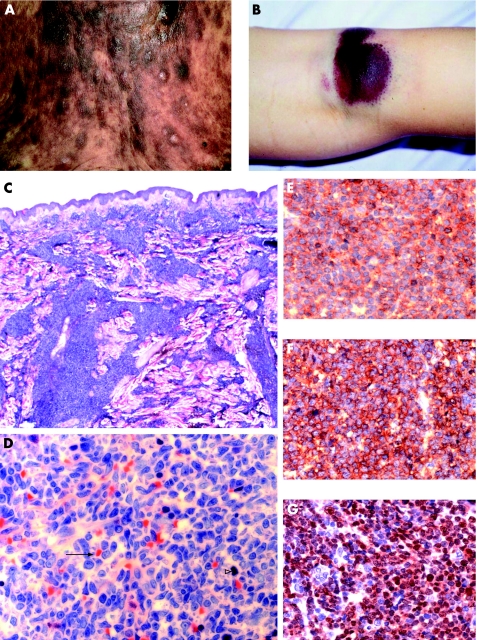
Figure 1 Morphological and immunophenotypic features of CD4+/CD56+ haematodermic neoplasm (group 1). (A) The trunk of a patient with generalised red to violet‐brown plaques and nodules (case 6). (B) Bruise‐like lesion on the right knee (case 3). (C and D) Histological findings of skin biopsy show dense infiltration of medium‐sized blastoid tumour cells without angiocentric growth pattern, and frequent atypical mitoses (solid arrow) and massive erythrocyte extravasation (open arrowhead). (E) Strong membranous staining of CD56. (F) Strong membranous staining of CD123. (G) Strong nuclear staining of TdT.
Histomorphology
The typical characteristics were diffuse monomorphous infiltrates of medium‐sized cells separated from the epidermis by a grenz zone. Perivascular patchy infiltrates and an Indian file pattern were occasionally observed. Angiotropism was uncommon (fig 1 C,D).
Immunophenotype
CD4 and CD56 as well as CD43 were expressed by the atypical cells in all cases investigated. The markers characteristic for B/T cell lineage (CD20/CD3), as well as for cytotoxic cell markers TIA1 and GrB, were negative like CD30. CD123 was positive in 6/6 cases where frozen material was available. TCL1 expression (T cell leukaemia antigen) was positive in 4/4 cases investigated. TdT was positive in 5/13 cases. The myelomonocytic markers CD33, MPO (myeloperoxidase), and CD68 were not expressed in 20/20 cases. Tumour cells of all cases were EBV‐negative as shown by the absence of LMP‐1 (latent membrane protein) and EBNA‐2 (fig 1E–G).
Genotype
A clonal TCR‐gamma gene rearrangement was not detectable in any of the 15/20 cases investigated, which excluded T lymphocytes as the precursor cell.
Group 2: skin infiltration of CD56+ AML
Clinical features
Three men and one woman (mean age 62 years, range 48–92 years) were investigated in this group. Three patients presented with solitary fast‐growing tumours (fig 2A,B), while one patient presented with multiple red to violet tumour nodules. At initial presentation, 2/4 (50%) patients had concurrent bone marrow, lymph node, and blood involvement. All patients died after a median survival of 11 months (range 3–60 months, 95% CI 0 to 42 months). Notably, the only patient who received allogenic bone marrow transplantation had the longest survival time of 60 months.
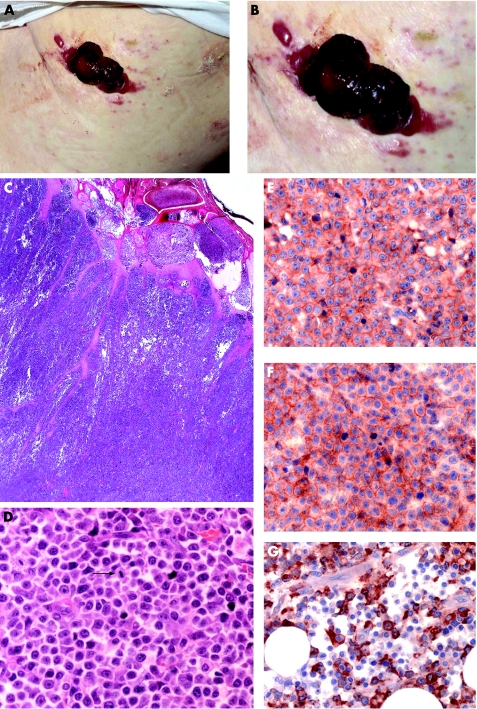
Figure 2 Morphological and immunophenotypic features of cutaneous infiltration of CD56+ AML (group 2). (A and B) Haemorrhagic tumour on the trunk (case 22). (C and D) Histology revealed diffuse infiltration of medium‐sized blast like cells with numerous mitoses (arrow). (E) Strong expression of CD4. (F) Strong expression of CD56. (G) Strong expression of MPO.
Histomorphology
The tumour cells were monomorphous and medium to large‐sized with round nuclei and fine diffuse chromatin. The infiltrate was located mainly in the middle and deep dermis, sparing the subepidermal region, and infiltrated the collagen bundles (fig 2C,D).
Immunophenotype
The neoplastic cells were negative for CD3, CD30 and TIA, but positive for CD4, CD33, CD43, CD56, CD68 and MPO. Interestingly, CD123 was positive in one of the two cases investigated (fig 2E–G). TCL1 was expressed in 2/3 cases. The tumour cells were negative for LMP‐1 and EBNA and thus EBV‐negative.
Genotype
A clonal TCR‐gamma gene rearrangement was not detectable in any of the four cases, which excluded T lymphocytes as the cell of origin.
Group 3: nasal‐type extranodal NK/T cell lymphoma
Clinical features
This category included five patients (three men, two women; mean age 72 years, range 58–73 years). The patients presented either with a fast‐growing, solitary tumour on the arm or trunk (2/5) (fig 3A) or with red to brown‐violet nodules on the trunk that tended to disseminate (3/5). One patient showed lymph‐node and bone‐marrow involvement at presentation (case 25). The disease was primarily cutaneous in four of the five cases, but secondary internal involvement included the palate in two cases (fig 3B), the lymph nodes in two, and the liver and bone marrow in one case each. Four patients died shortly after therapy with a median survival of 7 months (range 2–72 months, 95% CI 3 to 12 months). One patient is still alive without extracutaneous disease.
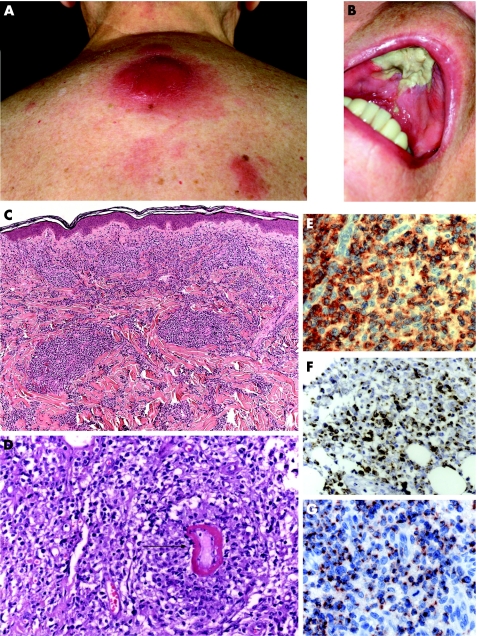
Figure 3 Morphological and immunophenotypic features of extranodal NK/T‐cell lymphoma (group 3). (A) Solitary violets tumour on the back; and in addition, (B) infiltration and destruction of the hard palate leading to extensive necrosis (case 29). (C and D) Histological findings show beneath a free grenz zone a prominent angiocentric growth of medium to large blastic lymphocytes, focally with invasion of the blood vessel (arrow). (E) Strong membranous staining of CD8. (F) Cytoplasmic expression of cytotoxic molecules (Gran B). (G) Cytoplasmic expression of cytotoxic molecules (TIA‐1) (G).
Histomorphology
All cases showed a diffuse, angiocentric and angiodestructive pattern of medium to large‐sized atypical lymphocytes (fig 3C,D). Epidermotropism was also seen in 3/4 cases. Coagulative necrosis and apoptotic cells were common.
Immunophenotype
In 5/5 cases, CD56 and cytotoxic markers (TIA1, GrB) were expressed by the atypical cells (fig 3E–G), whereas markers typical for B cells (CD20), as well as for the myelomonocytic lineage (CD33, MPO, CD68) were negative. Markers of EBV‐infection (LMP‐1, EBNA‐2) were expressed by the tumour cells in 3/4 cases. Atypical cells lacked the expression of CD4, CD8, and CD30 and were positive for cytoplasmic CD3 and/or CD3ε in two cases.
Genotype
A clonal TCR‐gamma gene rearrangement was detectable in one of the four cases investigated.
Group 4: “classical” primary cutaneous T cell lymphomas with CD56 co‐expression
Clinical features
This group comprised five patients (three men and two women; mean age 54.4 years, range 7–84 years). Two patients presented with the typical recurrent papules and nodules seen in cases of lymphomatoid papulosis (fig 5A); one case displayed generalised hypo‐ and hyperpigmented lesions of mycosis fungoides. Two patients showed subcutaneous tumours typical of panniculitis‐like T‐cell lymphoma. Tumours were of the primary cutaneous type in all cases, and systemic spread was not reported. All patients were alive at the time of evaluation (median follow‐up time 62 months).
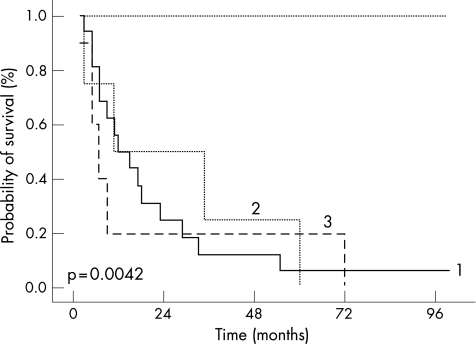
Figure 5 Prognosis of CD56+ haematolymphoid neoplasm. Overall survival showed by Kaplan–Meier survival curves, given by different types of cutaneous CD56+ neoplasm. (1) CD4+/CD56+ haematodermic neoplasm (n = 20). (2) Cutaneous CD56+ AML (n = 4). (3) Extranodal NK/T‐cell lymphoma (n = 5). (4) “Classical cutaneous T cell lymphoma” with co‐expression of CD56 (n = 5) (log‐rank test p = 0.0042).
Morphology and immunophenotype
CD56 expression of the atypical cells was seen in all cases. Four of five cases displayed a cytotoxic phenotype with expression of CD8 and/or TIA‐1 and GrB (fig 4B–D). The two cases of subcutaneous panniculitis‐like T‐cell lymphoma displayed an α/β‐phenotype. Case 30 with lymphomatoid papulosis had lost CD3, as well as CD4 and TCL1. The tumour cells of all four cases displayed no EBV infection (LMP‐1−, EBNA‐2−).
Figure 4 Morphological and immunophenotypic features of lymphomatoid papulosis with co‐expression of CD56. (A) Grouped papules on the right abdomen (case 30). (B) Histological findings are large atypical lymphocytes (open arrowhead) embedded in a mixed inflammatory infiltrate with eosiniophilic granulocytes (closed arrow). (C) Immunohistology revealed expression of CD30. (D) Immunohistology revealed expression of CD56.
Genotype
A clonal TCR gene rearrangement was detectable in all three cases investigated, indicating the T‐cell origin of the tumour cells.
CD56 expression in haematological cutaneous malignancies as an indicator of an unfavourable prognosis
Patients in groups 1–3 had an adverse clinical outcome, whereas patients in group 4 displayed an excellent clinical course (p = 0.0042; log‐rank test, fig 5). All 29 patients in groups 1–3, with the exception of one patient, died of disease (median survival probability 11 months, 95% CI 2 to 72 months). In contrast, all patients in group 4 were alive at the last follow‐up (5/5, 100%).
Discussion
The spectrum of cutaneous lymphomas expressing CD56 has been heterogeneous with respect to clinical presentation and course, morphology and immunohistochemical phenotype, as well as the association with EBV infection. To classify these neoplasms more precisely, we evaluated the clinical and histological/immunophenotypical characteristics of a large series of CD56+ haematological cases (n = 34) presenting in the skin. Based on the data obtained, we identified four groups which show significant differences in clinical behaviour and prognosis.
Despite their different cellular origin, CD56+ cases of haematodermic neoplasm/blastic NK‐cell lymphoma (group 1), of AML skin infiltration (group 2) and of extranodal NK/T‐cell lymphoma nasal type (group 3) are characterised by their extremely poor clinical outcome. This is in sharp contrast to the very favourable clinical outcome of patients with “classical” CD56+ primary cutaneous T‐cell lymphoma (group 4).
Haematodermic neoplasms (blastic NK‐cell lymphomas) (group 1) represent the largest group of our series (20/34, 56%), with a striking male dominance (15/20, 83%). Clinically, all patients experienced an aggressive clinical course regardless of whether they presented with localised or disseminated disease (median survival 12 months). This observation is in line with previous studies and is independent of the therapeutic regimens used.18,19
The origin of the CD4+/CD56+ tumour cells of the so‐called haematodermic neoplasms/blastic NK‐cell lymphomas is the subject of a longstanding debate. NK cell precursor, a myelomonocytic precursor or a mixed NK/myelomonocytic precursor have been proposed.20,21,22,24 Petrella et al recently suggested a derivation from pDC, as indicated by expression of the interleukin‐3 receptor alpha subunit (CD123).25,26 Moreover, the same group also showed TCL1 expression in a larger series of CD4+/CD56+ haematodermic neoplasms, thus excluding NK cells as the origin of this entity.7
The pDC origin of CD4+/CD56+ haematodermic neoplasms was furthermore supported by Hallermann et al and Urosevic et al, who showed the co‐expression of CD123 and “blood dendritic cell antigen” (BDCA‐2) in nearly all investigated cases of cutaneous CD4+/CD56+ haematodermic neoplasm.27,28,29 BDCA‐2 is a C‐type lectin transmembrane glycoprotein that internalises antigen for presentation to T‐cells and is specifically expressed by human pDC.29 The investigation of a newly‐generated cell line (CAL‐1) derived from a blastic NK‐cell lymphoma revealed further convincing results for the pDC origin of this entity.30
The second group (4/34) of cutaneous CD56+ neoplasms represents cutaneous infiltrates of acute myelomonocytic leukaemia (AML), as evidenced by their additional expression of myelomonocytic markers such as CD33, CD68 and MPO.17 The morphological and clinical features are highly similar to the cases of blastic NK‐cell lymphoma of group 1. This includes the observation that allogenic bone marrow transplantation appears to be the most beneficial therapeutic option. These concordant features suggest a possible relation of both neoplasms, which is supported by the fact that in some cases, patients with haematodermic neoplasms/blastic NK cell lymphoma develop myeloid or myelomonocytic leukaemia as also shown in 2/20 patients of groups 1 and 2.31,32
Recently several studies have shown that CD56+ expression on AML tumour cells appears to be associated with a shorter period of complete remission and reduced survival as compared to CD56− cases.33,34 This is in harmony with the finding that CD56+ myeloid leukaemia displays more frequently an extramedullary involvement (skin, lymph node) at initial presentation, suggesting that CD56+ myeloid leukaemia might constitute a distinct biological and clinical disease entity.24,35,36
The third group (5/34) identified among the CD56+ cutaneous neoplasms consists of skin involvement of nasal type extranodal NK/T‐cell lymphoma. The clinical course of the five patients (80% men) resembled those in previous reports in that the development of skin lesions (fast‐growing tumour nodules) was most frequently followed by involvement of the nasopharyngeal region (40%) and lymph nodes (40%), with early dissemination to the bone marrow and liver (20%).37 Despite intensive combined chemotherapy, patients with cutaneous NK/T‐cell lymphoma have an extremely poor prognosis, as most patients die within several months after its onset as also shown in our patients, who died after a median survival of only 7 months.
According to the WHO classification, extranodal NK/T‐cell lymphoma is nearly always associated with EBV infection, irrespective of the ethnic origin of the patients, suggesting a pathogenic role for the virus.17,38,39,40,41,42 However, we could not detect an EBV infection of tumour cells in 25% of the cases. This is in agreement with a recent series of primary cutaneous NK/T‐cell lymphomas of Asian and Caucasian patients, which were EBV‐negative in most cases (17/52, 33%).48,49,50 Interestingly, the histological features of extranodal NK/T‐cell lymphomas are indistinguishable among the various sites of involvement.
The cellular origin of the CD4+/CD56+ cells in extranodal NK/T‐cell lymphoma has not been completely clarified. In the majority of extranodal NK/T‐cell lymphomas, T‐cell receptor genes are in germline configuration, consistent with a natural killer cell origin.43,44,45,46,47 Conversely, clonal TCR‐receptor gene rearrangement as evidence for a T‐cell origin could be detected in nearly 30% of the investigated cases of cutaneous extranodal NK/T‐cell lymphoma.48,49,50 The evidence to date suggests that the postulated counterparts of extranodal NK/T‐cell lymphoma are probably both cell types, activated NK cells and cytotoxic T lymphocytes, indicating a close relationship to the precursor cell. However, the presence or absence of EBV or TCR rearrangement in extranodal NK/T cell lymphomas seems to have no prognostic significance.
The fourth group (5/34) represents cutaneous neoplasms with a co‐expression of the CD56 molecule but not included in the other groups. All cases of group 4 were cutaneous T‐cell lymphomas (CTCL) such as lymphomatoid papulosis (n = 2), CD8+ subcutaneous panniculitis‐like T‐cell lymphoma with an alpha/beta‐phenotype (n = 2) and mycosis fungoides (n = 1). However, besides the typical histological/immunohistochemical and molecular features for these lymphomas, an atypical CD56 expression of the tumour cells was detectable.5
The most striking feature of the cases of group 4 is their excellent clinical outcome (all patients alive: follow‐up time median 62 months) when compared to the clinical course of the CD56+ cases of the other three groups. Considering the heterogeneity and small number of patients in group 4, our results suggests that the expression of CD56 cannot be regarded as a general marker for adverse prognosis identifying the need to discriminate among the patients suffering from CD56+ neoplasms. However, cases previously termed as subcutaneous panniculitis T‐cell lymphoma with a gamma/delta phenotype are now described in the recent WHO–EORTC classification as cutaneous gamma/delta‐T cell lymphoma and should be distinguished from subcutaneous panniculitis like T‐cell lymphoma. Most patients with a cutaneous gamma/delta‐T cell lymphoma have an aggressive disease and are resistant to multiagent chemotherapy and/or radiation therapy, showing a median survival of only 15 months.5
Take‐home messages
Cutaneous lymphomas expressing CD56, a neural cell adhesion molecule, are characterised in most cases by a highly aggressive clinical course and poor prognosis.
Aggressive types of CD56‐positive neoplasms of the skin are represented by haematodermic neoplasm, skin infiltration as the first manifestation of CD56+ acute myeloid leukaemia, and nasal‐type extranodal NK/T‐cell lymphoma.
Cutaneous T‐cell lymphoma (CTCL), for example panniculitis like T‐cell lymphoma, may also show expression of the CD56 molecule, which is not associated with an aggressive clinical course.
Therefore, it is clinically very important to separate CD56+ CTCL from the remaining CD56+ haematological disorders.
In conclusion, our findings indicate that CD56+ cutaneous lymphoproliferative disorders comprise a spectrum of different entities with specific clinical and biological features preferentially occurring in men (26/34, 76%). Most of these entities are associated with an aggressive clinical course and poor outcome with a median survival time of only 7–12 months. However, CD56 expression by itself is not a prognostic marker since several types of “classical CTCL” cases may also show a co‐expression of CD56. The extreme prognostic difference among the patients with cutaneous CD56+ neoplasms requires a precise diagnostic evaluation in order to identify those patients who presumably benefit from bone marrow transplantation. Because of the rarity of the diseases described in this study and the small number of patients, especially in groups 2–4, multicentre trials should be initiated to determine the most appropriate treatment strategies.
Acknowledgements
We thank Patricia Zambon for her help in preparing this manuscript.
Abbreviations
AML - acute myelomonocytic leukaemia
CTCL - cutaneous T cell lymphoma
EBV - Epstein‐Barr virus
EORTC - European Organisation for Research and Treatment of Cancer
NK - natural killer
pDC - plasmacytoid dendritic cells
TCR - T‐cell receptor
Footnotes
Competing interests: None declared.
References
- 1.Lanier L L, Chang C, Azuma M.et al Molecular and functional analysis of human natural killer cell‐associated neural cell adhesion molecule (N‐CAM/CD56). J Immunol 19911464421–4426. [PubMed] [Google Scholar]
- 2.Kern W F, Spier C M, Hanneman E H.et al Neural cell adhesion molecule‐positive peripheral T‐cell lymphoma: a rare variant with a propensity for unusual sites of involvement. Blood 1992792434–2437. [PubMed] [Google Scholar]
- 3.Thoulouze M I, Lafage M, Schachner M.et al The neural cell adhesion molecule is a receptor for rabies virus. J Virol 1998727181–7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin J D, Hercend T, Beveridgeet al Characterization of an antigen expressed by human natural killer cells. J Immunol 19831302947–2951. [PubMed] [Google Scholar]
- 5.Willemze R, Jaffe E S, Burg G.et al WHO‐EORTC classification for cutaneous lymphomas. Blood 20051053768–3785. [DOI] [PubMed] [Google Scholar]
- 6.Chim C S, Ooi G C, Shek T W.et al Lethal midline granuloma revisited: nasal T/natural‐killer cell lymphoma. J Clin Oncol 1999171322–1325. [DOI] [PubMed] [Google Scholar]
- 7.Petrella T, Meijer C J, Dalac S.et al TCL1 and CLA expression in agranular CD4/CD56 hematodermic neoplasms (blastic NK‐cell lymphomas) and leukemia cutis. Am J Clin Pathol 2004122307–313. [DOI] [PubMed] [Google Scholar]
- 8.Reichard K K, Burks E J, Foucar M K.et al CD4(+) CD56(+) lineage‐negative malignancies are rare tumours of plasmacytoid dendritic cells. Am J Surg Pathol 2005291274–1283. [DOI] [PubMed] [Google Scholar]
- 9.Kwong Y L. Natural killer‐cell malignancies: diagnosis and treatment. Leukemia 2005192186–2194. [DOI] [PubMed] [Google Scholar]
- 10.Ng A P, Lade S, Rutherford T.et al Primary cutaneous CD4+/CD56+ hematodermic neoplasm (blastic NK‐cell lymphoma): a report of five cases. Haematologica 200691143–144. [PubMed] [Google Scholar]
- 11.Mraz‐Gernhard S, Natkunam Y, Hoppe R T.et al Natural killer/natural killer‐like T‐cell lymphoma, CD56+, presenting in the skin: an increasingly recognized entity with an aggressive course. J Clin Oncol 2001192179–2188. [DOI] [PubMed] [Google Scholar]
- 12.Bekkenk M W, Jansen P M, Meijer C J.et al CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol 2004151097–1108. [DOI] [PubMed] [Google Scholar]
- 13.Urosevic M, Kamarashev J, Burg G.et al Primary cutaneous CD8+ and CD56+ T‐cell lymphomas express HLA‐G and killer‐cell inhibitory ligand, ILT2. Blood 20041031796–1798. [DOI] [PubMed] [Google Scholar]
- 14.Assaf C, Hummel M, Dippel E.et al High detection rate of T‐cell receptor beta chain rearrangements in T‐cell lymphoproliferations by family specific polymerase chain reaction in combination with the GeneScan technique and DNA‐sequencing. Blood 200096640–646. [PubMed] [Google Scholar]
- 15.Whittaker S J, Smith N P, Jones R R.et al Analysis of beta, gamma, and delta T‐cell receptor genes in mycosis fungoides and Sezary syndrome. Cancer 1991681572–1582. [DOI] [PubMed] [Google Scholar]
- 16.Nie H H, Hadlai H, Jenkins J G.et alSPSS (Statistical Package for the Social Sciences). New York, NY: McGraw‐Hill, 1979
- 17.Jaffe E S, Harris N L, Stein H, Vardiman J W. eds. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues Lyon: IARC Press, 2001
- 18.Jacob M C, Chaperot L, Mossuz P.et al CD4+ CD56+ lineage negative malignancies: a new entity developed from malignant early plasmacytoid dendritic cells. Haematologica 200388941–955. [PubMed] [Google Scholar]
- 19.Feuillard J, Jacob M C, Valensi F.et al Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood 2002991556–1563. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura S, Suchi T, Koshikawa T.et al Clinicopathologic study of CD56 (NCAM)‐positive angiocentric lymphoma occurring in sites other than the upper and lower respiratory tract. Am J Surg Pathol 199519284–296. [DOI] [PubMed] [Google Scholar]
- 21.Drenou B, Lamy T, Amiot L.et al CD3‐ CD56+ non‐Hodgkin's lymphomas with an aggressive behavior related to multidrug resistance. Blood 1997892966–2974. [PubMed] [Google Scholar]
- 22.Emile J F, Boulland M L, Haioun C.et al CD5‐CD56+ T‐cell receptor silent peripheral T‐cell lymphomas are natural killer cell lymphomas. Blood 1996871466–1473. [PubMed] [Google Scholar]
- 23.Bagot M, Bouloc A, Charue D.et al Do primary cutaneous non‐T non‐B CD4+CD56+ lymphomas belong to the myelo‐monocytic lineage? J Invest Dermatol 19981111242–1244. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki R, Yamamoto K, Seto M.et al CD7+ and CD56+ myeloid/natural killer cell precursor acute leukemia: a distinct hematolymphoid disease entity. Blood 1997902417–2428. [PubMed] [Google Scholar]
- 25.Petrella T, Comeau M R, Maynadie M.et al ‘Agranular CD4+ CD56+ hematodermic neoplasm' (blastic NK‐cell lymphoma) originates from a population of CD56+ precursor cells related to plasmacytoid monocytes. Am J Surg Pathol 200226852–862. [DOI] [PubMed] [Google Scholar]
- 26.Petrella T, Bagot M, Willemze R.et al Blastic NK‐cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms). Am J Clin Pathol 2005123662–675. [PubMed] [Google Scholar]
- 27.Hallermann C, Middel P, Griesinger F.et al CD4+ CD56+ blastic tumour of the skin: cytogenetic observations and further evidence of an origin from plasmocytoid dendritic cells. Eur J Dermatol 200414317–322. [PubMed] [Google Scholar]
- 28.Urosevic M, Conrad C, Kamarashev J.et al CD4+CD56+ hematodermic neoplasms bear a plasmacytoid dendritic cell phenotype. Hum Pathol 2005361020–1024. [DOI] [PubMed] [Google Scholar]
- 29.Dzionek A, Fuchs A, Schmidt P.et al BDCA‐2, BDCA‐3, and BDCA‐4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol 20001656037–6046. [DOI] [PubMed] [Google Scholar]
- 30.Maeda T, Murata K, Fukushima T.et al A novel plasmacytoid dendritic cell line, CAL‐1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol 200581148–154. [DOI] [PubMed] [Google Scholar]
- 31.Khoury J D, Medeiros L J, Manning J T.et al CD56(+) TdT(+) blastic natural killer cell tumour of the skin: a primitive systemic malignancy related to myelomonocytic leukemia. Cancer 2002942401–2408. [DOI] [PubMed] [Google Scholar]
- 32.Kazakov D V, Mentzel T, Burg G.et al Blastic natural killer‐cell lymphoma of the skin associated with myelodysplastic syndrome or myelogenous leukaemia: a coincidence or more? Br J Dermatol 2003149869–876. [DOI] [PubMed] [Google Scholar]
- 33.Baer M R, Stewart C C, Lawrence D.et al Expression of the neural cell adhesion molecule CD56 is associated with short remission duration and survival in acute myeloid leukemia with t(8;21)(q22;q22). Blood 1997901643–1648. [PubMed] [Google Scholar]
- 34.Murray C K, Estey E, Paietta E.et al CD56 expression in acute promyelocytic leukemia: a possible indicator of poor treatment outcome? J Clin Oncol 199917293–297. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki R, Nakamura S, Suzumiya J.et al Blastic natural killer cell lymphoma/leukemia (CD56‐positive blastic tumour): prognostication and categorization according to anatomic sites of involvement. Cancer 20051041022–1031. [DOI] [PubMed] [Google Scholar]
- 36.Kaddu S, Beham‐Schmid C, Zenahlik P.et al CD56+ blastic transformation of chronic myeloid leukemia involving the skin. J Cutan Pathol 199926497–503. [DOI] [PubMed] [Google Scholar]
- 37.Chim C S, Ooi G C, Shek T W.et al Lethal midline granuloma revisited: nasal T/natural‐killer cell lymphoma. J Clin Oncol 1999171322–1325. [DOI] [PubMed] [Google Scholar]
- 38.Chan J K, Sin V C, Wong K F.et al Nonnasal lymphoma expressing the natural killer cell marker CD56: a clinicopathologic study of 49 cases of an uncommon aggressive neoplasm. Blood 1997894501–4513. [PubMed] [Google Scholar]
- 39.Chang S E, Huh J, Choi J H.et al Clinicopathological features of CD56+ nasal‐type T/natural killer cell lymphomas with lobular panniculitis. Br J Dermatol 2000142924–930. [DOI] [PubMed] [Google Scholar]
- 40.Ko Y H, Ree H J, Kim W S.et al Clinicopathologic and genotypic study of extranodal nasal‐type natural killer/T‐cell lymphoma and natural killer precursor lymphoma among Koreans. Cancer 2000892106–2116. [DOI] [PubMed] [Google Scholar]
- 41.Ahn S J, Jang K A, Choi J H.et al Nasal and nasal type CD56+ natural killer cell/T‐cell lymphoma: a case with rapid progression to bone marrow involvement. Br J Dermatol 20001421021–1025. [DOI] [PubMed] [Google Scholar]
- 42.Kluin P M, Feller A, Gaulard P.et al Peripheral T/NK‐cell lymphoma: a report of the IXth Workshop of the European Association for Haematopathology. Histopathology 200138250–270. [DOI] [PubMed] [Google Scholar]
- 43.Spits H, Lanier L L, Phillips J H. Development of human T and natural killer cells. Blood 1995852654–2670. [PubMed] [Google Scholar]
- 44.Lanier L L, Le A M, Civin C I.et al The relationship of CD16 (Leu‐11) and Leu‐19 (NKH‐1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol 19861364480–4486. [PubMed] [Google Scholar]
- 45.Bagot M, Bouloc A, Charue D.et al Do primary cutaneous non‐T non‐B CD4+CD56+ lymphomas belong to the myelo‐monocytic lineage? J Invest Dermatol 19981111242–1244. [DOI] [PubMed] [Google Scholar]
- 46.Petrella T, Dalac S, Maynadie M.et al CD4+ CD56+ cutaneous neoplasms: a distinct hematological entity? Groupe Francais d'Etude des Lymphomes Cutanes (GFELC). Am J Surg Pathol 199923137–146. [DOI] [PubMed] [Google Scholar]
- 47.Feuillard J, Jacob M C, Valensi F.et al Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood 2002991556–1563. [DOI] [PubMed] [Google Scholar]
- 48.Takeshita M, Yoshida K, Suzumiya J.et al Cases of cutaneous and nasal CD56 (NCAM)‐positive lymphoma in Japan have differences in immunohistology, genotype, and etiology. Hum Pathol 1999301024–1034. [DOI] [PubMed] [Google Scholar]
- 49.Natkunam Y, Smoller B R, Zehnder J L.et al Aggressive cutaneous NK and NK‐like T‐cell lymphomas: clinicopathologic, immunohistochemical, and molecular analyses of 12 cases. Am J Surg Pathol 199923571–581. [DOI] [PubMed] [Google Scholar]
- 50.Miyamoto T, Yoshino T, Takehisa T.et al Cutaneous presentation of nasal/nasal type T/NK cell lymphoma: clinicopathological findings of four cases. Br J Dermatol 1998139481–487. [DOI] [PubMed] [Google Scholar]