Abstract
Background
Roux‐en‐Y gastric bypass surgery provides a novel human model to investigate small bowel mucosal innate immunity, in which there is loss of gastric acid‐mediated protection against orally‐acquired microorganisms.
Aim
To study changes in jejunal mucosal immunoreactivity of human defensin (HD)‐5, an antimicrobial peptide normally produced by Paneth cells.
Methods
Mucosal samples were obtained from 18 female patients (24–54 years), from the same segment of jejunum during and after gastric bypass surgery. Samples were used for bacterial culture and immunohistochemistry using anti‐HD‐5 antibody. The number of immunoreactive cells per crypt and villus were determined and expressed as mean (SD).
Results
No bacteria were cultured from any of the perioperative jejunal samples but colonies of bacteria normally present in the pharynx were identified during culture of all postoperative jejunal biopsy specimens (1–>100 colonies). Paneth cell numbers per crypt were unchanged after gastric bypass (4.16 (0.71) vs 4.24 (0.78)). However, following surgery, there was an increase in HD‐5‐positive intermediate cells per crypt (0.25 (0.41) vs 1.12 (0.66), p<0.01), HD‐5 staining enterocytes per crypt (0.03 (0.09) vs 1.38 (1.10), p<0.01), HD‐5 staining material in the crypt lumen (crypt lumens: 5.0% (10.9%) vs 68.1% (27.9%), p<0.01) and HD‐5 immunoreactivity coating the luminal surface of villus enterocytes (villi sampled: 15.0% (31.0%) vs 67.5% (42.0%), p<0.01).
Conclusions
Bacteria normally resident in the pharynx were present in the proximal jejunal mucosa following Roux‐en‐Y gastric bypass surgery. After gastric bypass, there was increased secretion of HD‐5 and an increase in HD‐5 expressing intermediate cells and enterocytes in the crypt. The increase in HD‐5 expression in the jejunal mucosa following gastric bypass surgery is likely to be secondary to exposure to orally‐acquired microorganisms.
Keywords: antimicrobial peptide, innate immunity, intermediate cells, obesity, Paneth cells
The gastrointestinal tract may be frequently exposed to orally‐acquired microorganisms and a number of host protective factors are believed to normally avoid colonisation of the small intestine, where nutrient digestion and absorption takes place. Gastric acid is well known to represent an important component of antimicrobial defence. There is also increasing recognition of the role of antimicrobial peptides and proteins expressed by epithelial cells, especially Paneth cells, in the small intestine.1,2 Paneth cells are present at the base of crypts, below the stem cell zone. In addition to lysozyme3 and secretory phospholipase A2,4 Paneth cells also express antimicrobial peptides of the α‐defensin family.5,6 In humans, Paneth cells express two members of the α‐defensin family, human defensin (HD)‐5 and HD‐6, which are stored in granules present in the apical cytoplasm.
Roux‐en‐Y gastric bypass surgery is frequently undertaken for patients with severe obesity.7 In this operation, the jejunum is anastomosed to the oesophagus, via a small gastric pouch (fig 1). Thus, the stomach is bypassed by ingested food, to be deposited directly into the jejunum. At this site, there is also lack of secretions from the liver and pancreas, which are transported postoperatively by the proximal biliopancreatic limb to a more distal part of the small intestine. Thus, following Roux‐en‐Y gastric bypass surgery, microorganisms ingested with food will come into direct contact with the proximal small intestine. These individuals therefore represent a unique model in which interactions between orally‐acquired microorganisms and the human small intestine can be investigated.
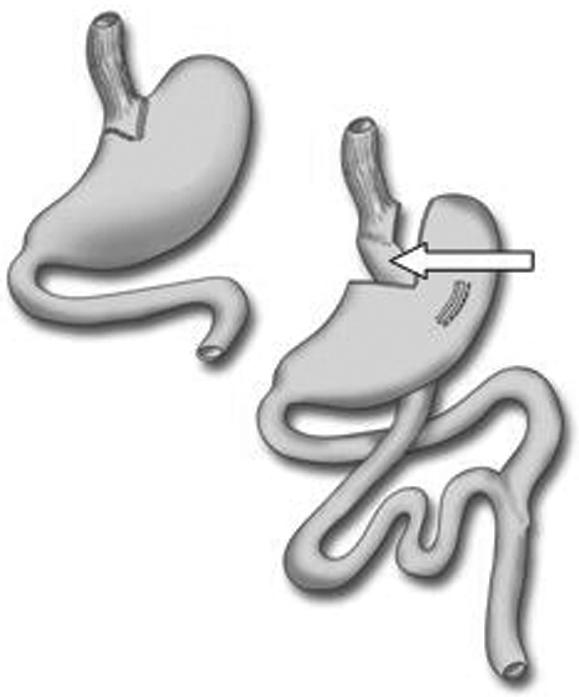
Figure 1 Principle of Roux‐en‐Y gastric bypass. Food and saliva pass directly into the small bowel without contact with gastric or duodenal juices. Gastric acid and bile do not enter the jejunum until at the Y‐shaped junction 20 cm distal to the ligament of Treitz. Mucosal biopsy specimens were taken from the proximal Roux‐limb (white arrow), a part of the jejunum normally situated 30 cm distal to ligament of Treitz.
In this report, we have studied mucosal samples from the proximal jejunum of patients before and after gastric bypass surgery for the presence of bacteria and expression of the antimicrobial molecules HD‐5 and lysozyme.
Materials and methods
Subjects
Eighteen female patients, median age 36 (24–54) years, body mass index (BMI) 46 (30–57) kg/m2 scheduled for a Roux‐en Y gastric bypass were recruited for this study, which was approved by the Ethics Committee at Uppsala University. All patients gave informed consent. Fourteen of the patients were having bariatric surgery for the first time and four patients (BMI 30, 30, 31 and 39, respectively) were operated on due to a failed restrictive procedure. The gastric bypass was performed either by hand‐assisted laparoscopy or through a short upper midline incision at the Department of Surgery, Uppsala, Sweden as previously described.8 In short, a small proximal gastric pouch (4×3 cm) was created along the lesser curve of the stomach using cutting linear staplers (EndoGIA II, TYCO, Norwalk, Connecticut, USA), totally dividing and excluding the main stomach. The small pouch was anastomosed to a 50 cm retrocolic, retrogastric Roux‐limb by a circular stapler (ILS 21, Ethicon Endo‐Surgery, Cincinnati, Ohio, USA). The small bowel continuity was maintained by a side‐to‐side entero‐enterostomy. Single doses of intravenous cefuroxime (1.5 g) and metronidazole (500 mg) were given in the operating room at the start of the procedure.
Tissue sampling
Jejunal mucosal samples were obtained from the proximal Roux limb during surgery using sterile forceps. The first sample was transferred to freezing media containing 20% glycerol and stored at −70°C for subsequent bacterial culture. Additional samples were taken and stored in 4% buffered formaldehyde for histology. Postoperative samples were taken endoscopically. At endoscopy, sterile biopsy forceps were used to take samples from the proximal Roux limb, from the same small bowel segment as before at 50 cm from the incisors (fig 1). The endoscopic examination was performed during the hospital stay (day 3–7) in 3 patients, at the first outpatient visit (day 22–57) in 13 patients and in the remaining 2 patients after 136 and 291 days, respectively. The samples were stored as above for subsequent bacterial culture and histology.
HD‐5 immunohistochemistry
Mucosal samples were embedded in paraffin wax. Sequential 5 μm thick sections were cut and stained with H&E. Immunohistochemistry was performed using antibodies to HD‐5,9 lysozyme (NeoMarkers, Fremont, California, USA) and intestinal trefoil factor. Immunohistochemistry was performed using a Vectastain Universal Elite ABC kit (Vector Laboratories, Burlingame, California, USA), according to manufacturer's instructions. Peroxidase activity was developed with Vector VIP reagent, and then washed with tap water. Following counterstaining with haematoxylin, tissue sections were dehydrated through graded ethanols and xylene, and then mounted using DPX.
Counts of HD‐5 staining cells
HD‐5 immunoreactive cells were analysed along 10 crypt–villus units in each section. Parts of the section studied were those in which the longitudinal axis of the lumen was aligned such that it could be seen from the villus tip to the crypt base. Paneth cells were defined as those HD‐5 staining cells in the base of the crypt, below the stem cell zone, with granular cytoplasm. Intermediate cells had a mucin component to the cytoplasm, as well as HD‐5 staining, and were recorded as located either in the crypt (above the stem cell zone) or in the villus. HD‐5 staining enterocytes had no mucin component, but stained positive for HD‐5 when compared to negative controls. These were recorded as being in the crypt or villus. HD‐5 staining of the microvilli border to the villus was recorded if present (when compared to negative controls) and HD‐5 staining in the crypt lumen was recorded if present (when compared to negative controls). These scores were given by one observer (DE), but the validity of the scores was assessed by a second observer (YM).
Immunoelectron microscopy
Serial 10 μm sections from paraffin wax embedded blocks were cut, and the presence of HD‐5 detected using the immunohistochemical technique described above, although peroxidase activity was developed using Vector DAB reagent. Negative controls on sequential sections were performed. After development of peroxidase activity, sections were fixed in 2.5% (v/v) glutaraldehyde in 0.1M cacodylate buffer pH 7.4, dehydrated in ascending grades of ethanol and infiltrated with a 1:1 mixture of epoxypropane:Taab resin. A resin filled capsule was inverted over the section in HD‐5 staining cells and allowed to polymerise at 60°C for 18 hours. This was repeated for the same region on the sequential section negative control. After removing the capsules, containing the embedded areas of tissue, 0.5 μm sections were cut and stained with 1% (w/v) toluene blue. Sections (80 nm) were cut from areas of interest (identified from the toluene blue stained sections), mounted on copper grids and stained with uranyl acetate. After washing in 50% (v/v) ethanol, the sections were further stained with Reynold's lead citrate before examining in a Jeol 1010 transmission electron microscope.
Culture of bacteria
The frozen samples were cultured at the end of the study. In the laboratory the biopsy specimens were thawed, homogenised and 0.1 ml was spread onto 5% blood agar and J‐agar (for anaerobic bacteria), respectively, and incubated at 35°C for 2 days. The agar plates were then analysed for bacterial growth using standard microbiology methods. The commensal and pathogenic flora were determined and quantified according to standard procedures, that is sparse (1–50 colony forming units/plate), medium (51–100 CFU/plate) and rich (>100 CFU/plate).
Statistical analysis
Histological scores are given as mean and standard deviation, patient data as median and range. Data were analysed using SPSS V.11.1 for Windows. Student's t‐test was used to compare paired sample means; p<0.05 was considered statistically significant.
Results
Subjects
All patients had an uneventful recovery and were discharged when tolerating solids, on postoperative day 6 (3–7). After one year, all patients were well; BMI was reduced from 46 (30–57) kg/m2 to 29 (22–37) kg/m2. No patient had diarrhoea, abdominal pain or frequent gastrointestinal infections.
Bacterial growth
After culture, no bacterial growth was seen from any of the 18 perioperative tissue samples. Sixteen postoperative tissue samples were cultured. All contained normal pharyngeal flora (i.e. alpha‐Streptococcus spp, Neisseria spp and Staphylococcus spp), with varying colony counts. No correlation was found between colony count and time following surgery that the tissue sample was taken. Candida albicans was identified in one postoperative sample (taken at day 291), but no other pathogens were seen (table 1). No anaerobic bacteria were cultured.
Table 1 Summary of bacterial cultures from jejunal samples taken at endoscopy between 3 and 291 days after Roux‐en‐Y gastric bypass surgery.
| No of colonies | No of patients |
|---|---|
| 1–50 | 10 |
| 51–100 | 5 |
| >100 | 1 |
All cultures showed normal pharyngeal flora and no pathogens were seen except Candida albicans in one sample (taken on day 291). No correlation was found between the number of colonies and time after surgery.
Immunohistological studies
H&E stained sections did not show any evidence of inflammation or structural abnormality in jejunal sections obtained at the time of, or after surgery. Preliminary studies using pre‐immune serum and anti‐HD‐5 anti‐serum pre‐absorbed with excess synthetic HD‐5 confirmed specificity of anti‐HD‐5 antibody.
Paneth cell numbers were not affected by gastric bypass
In jejunal sections taken before and after gastric bypass there was no significant difference in the number of Paneth cells, as defined by specific HD‐5 staining cells with a granular cytoplasm in the base of the crypt below the stem cell zone. The mean (SD) number of Paneth cells per crypt, on analysing 10 longitudinally orientated crypts from each section, over the 18 perioperative samples was 4.16 (0.71). Postoperatively, the mean (SD) number of Paneth cells per crypt for the 18 patients was 4.24 (0.78) (table 2).
Table 2 Cell staining after human defensin (HD)‐5 immunostaining.
| Cell type | Cell counts (mean (SD)) | p Value for paired differences | |
|---|---|---|---|
| Perioperative | Postoperative | ||
| Paneth cells | 4.16 (0.71) | 4.24 (0.78) | 0.66 |
| Intermediate cells (crypt) | 0.25 (0.41) | 1.12 (0.66) | <0.01 |
| Intermediate cells (villi) | 0.38 (0.72) | 0.25 (0.72) | 0.60 |
| HD‐5 staining enterocytes (crypt) | 0.03 (0.09) | 1.38 (1.10) | <0.01 |
| HD‐5 staining enterocytes (villi) | 0.01 (0.03) | 0.03 (0.08) | 0.38 |
| HD‐5 coating microvilli | 15% (31%) | 67.5% (42%) | <0.01 |
| HD‐5 staining crypt lumen | 5% (10.9%) | 68.1% (27.9%) | <0.01 |
Intermediate cells in the crypt are increased after gastric bypass
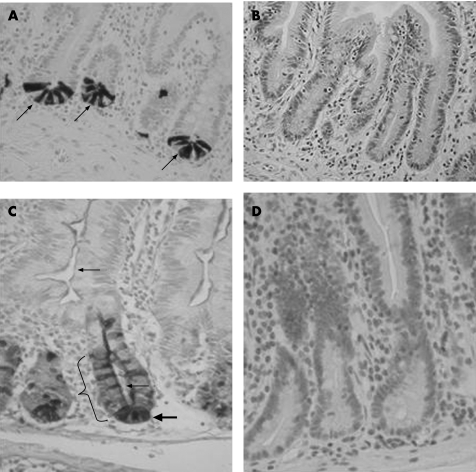
Intermediate cells, as defined by specific HD‐5 stained cells with an additional mucin component to the cytoplasm, in the crypt were increased from a mean (SD) of 0.25 (0.41) to 1.12 (0.66) following gastric bypass (p<0.01). There was no increase in intermediate cell numbers in the villi (mean 0.38 vs 0.25) (table 2 and fig 2A,B).
Figure 2 Human defensin‐5 immunostaining. (A) Perioperative sample where human defensin (HD)‐5 immunoreactive Paneth cells (arrows) with granular cytoplasm are seen in the base of jejunal crypts. (B) Control to fig 2A. No immunoreactivity is seen when pre‐immune serum is used instead of polyclonal anti‐HD‐5 anti‐serum. (C) Postoperative sample (same patient as fig 2A,B). Jejunal crypts show a marked increase in HD‐5 immunoreactive cells (bracket); there is HD‐5 staining in the crypt lumen (lower small arrow), coating of the micro‐villi (upper small arrow), but Paneth cell numbers remain unchanged (large arrow). (D) Control to fig 2C. No immunoreactivity is seen when pre‐immune serum is used instead of polyclonal anti‐HD‐5 anti‐serum.
HD‐5 staining enterocytes in the crypt are increased after gastric bypass
HD‐5 staining enterocytes, as defined by specific HD‐5 staining cells lining the crypt, above the stem cell zone, without mucinous features of goblet cells, were increased from mean (SD) 0.03 (0.09) to 1.38 (1.10) following gastric bypass surgery (p<0.01). There was no significant increase in HD‐5 staining enterocytes in the villi (0.01 vs 0.03) (table 2 and fig 2A,B).
HD‐5 expression in the lumen is increased after gastric bypass
HD‐5 coating on the microvilli was increased from 15% of villi sampled (10 villi per section of 18 patients) to 68% following gastric bypass surgery (p<0.01). HD‐5 staining in the crypt lumen was also increased from 5% to 68% of crypts sampled following gastric bypass surgery (table 2).
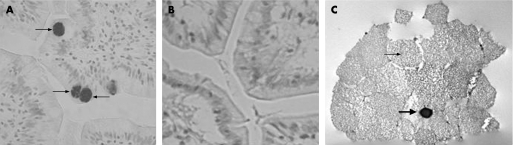
Intermediate cells contain large HD‐5 positive granules
High power light microscopy revealed that intermediate cells contain HD‐5 staining granules that are of greater size than granules in Paneth cells (fig 3A). These intermediate cells were further investigated by immunoelectron microscopy. These cells contain large, pale granules typical of the mucinous content seen in goblet cells, as well as smaller, electron dense granules that were DAB stained after immunohistochemistry with anti‐HD‐5 antibody (fig 3B).
Figure 3 Human defensin‐5 immunostaining in jejunal tissue. (A) Intermediate cells contain large, human defensin (HD)‐5 immunoreactive, granules (arrows). (B) No staining is seen when pre‐immune serum is used instead of anti‐HD‐5 anti‐serum. (C) Intermediate cell under transmission electron microscope. An electron dense, HD‐5 immunoreactive, granule (large arrow) is seen within the pale mucinous granules typical of a goblet cell (small arrow).
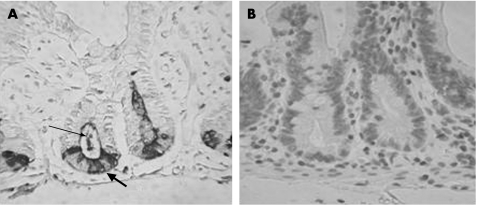
Lysozyme expressing cells are not increased following gastric bypass surgery
Immunohistochemistry was performed on representative sections using anti‐lysozyme antibody. Sections which showed marked increase in HD‐5 expression in the upper part of the crypt following gastric bypass did not show a similar increase in lysozyme expression (fig 4). However, in samples obtained following surgery, lysozyme‐immunoreactive coating was seen on the apical surface of epithelial cells and immunoreactive material was also seen in the crypt lumen.
Figure 4 Lysozyme immunostaining in postoperative jejunal samples. (A) Lysozyme immunoreactivity is confined to the Paneth cell zone in postoperative samples (large arrow), unlike human defensin‐5 immunoreactivity which spreads to the upper crypt (see fig 2C). Lysozyme staining is also seen in the crypt lumen (small arrow). (B) No staining is seen when non‐immune serum is used instead of anti‐lysozyme antiserum.
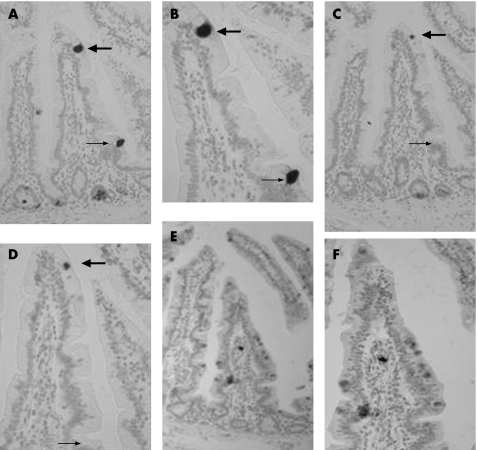
Many intermediate cells express lysozyme as well as HD‐5
Sequential 5 μm sections of jejunal tissue showed that a number of HD‐5 positive intermediate cells also immunoreactive for lysozyme (fig 5). Sequential sections from three representative samples showed that of 165 HD‐5 positive intermediate cells, 99 (60%) also expressed lysozyme.
Figure 5 Sequential 5 μm section immunohistochemistry with anti‐human defensin (HD)‐5 antibody (A, B), anti‐lysozyme antibody (C, D) and anti‐intestinal trefoil factor antibody (E, F) seen at low (A, C, E) and high (B, D, F) magnification. An intermediate cell on the villus tip (large arrow) is seen to express HD‐5 and lysozyme, whereas an intermediate cell towards the villus base expresses HD‐5 but not lysozyme (small arrow). The lack of Paneth cell staining using the rabbit polyclonal anti‐intestinal trefoil factor antibody (E, F) in these sequential sections also serves as a control for the HD‐5 and lysozyme polyclonal antisera.
Discussion
Our study has shown that following gastric bypass surgery, there is increased expression of HD‐5 in samples of jejunal mucosa that come in direct contact with ingested nutrients, without prior exposure to antimicrobial activities in the gastric juice and biliary and pancreatic secretions in the proximal small intestinal lumen. Thus following bypass surgery, there was an increase in the number of cells expressing HD‐5 and also HD‐5 immunoreactive material in the crypt lumen and coating the surface of enterocytes on the villi. The latter is likely to reflect increased secretion of HD‐5 by Paneth cells, intermediate cells and some enterocytes.
Under normal circumstances, the lumen of the jejunum would be devoid of microorganisms, as illustrated by lack of bacterial growth during culture of the perioperative mucosal samples. By contrast, bacteria normally resident in the pharynx were cultured from all postoperative jejunal mucosal samples studied. It is likely that the increase in HD‐5 expression in the postoperative jejunal epithelium is due to the presence of the identified bacteria. It is also possible that exposure (which may be transient) to other bacteria (or their products) associated with ingested nutrients contribute to alterations in HD‐5 expression. The increase in expression of HD‐5 may occur either following direct interaction of the bacteria (or their products) with epithelia cells, or indirectly via inflammatory/immune mediators induced by the bacteria. Secretion of antimicrobial α‐defensins (cryptdins) by Paneth cell‐containing murine small intestinal crypts occurs after exposure to bacteria or their products, such as lipopolysaccharide, lipoteichoic acid, lipid A and muramyl dipeptide.10 Such responses would be expected to occur via expression of receptors that mediate host recognition of microbial products.11,12,13,14 Murine small intestinal infection with the parasite Trichinella spiralis also induces Paneth cell degranulation.15 Concentrations of murine cryptdins in the crypt microenvironment can be in the region of 15–100 mg/ml.10 Such high concentrations of the α‐defensins, as may also occur for HD‐5 in postoperative samples of the current study, could play an important role in protecting stem cells, which are located above Paneth cells.16
HD‐5 expresses a broad spectrum of antimicrobial activity against bacteria17,18,19 and is also active against viruses such as the sexually transmitted human papillomaviruses.20 In addition to Paneth cell degranulation, infection with Trichinella spiralis also induces an increase in the number of α‐defensin‐expressing Paneth and intermediate cells in the small intestine.15 By contrast, Salmonella typhimurium has been reported to inhibit murine Paneth cell expression of α‐defensins and lysozyme.21 In humans, reduced expression of HD‐5 may be associated with susceptibility to intestinal infection.22
Take‐home messages
In the proximal jejunum following Roux‐en‐Y gastric bypass surgery, there is colonisation by bacteria normally resident in the pharynx.
There is also increased secretion of the antimicrobial peptide human defensin‐5 and lysozyme.
Cells other than Paneth cells express human defensin‐5.
It remains to be determined whether the bacteria isolated from the postoperative jejunal samples reflect their relative resistance to luminal HD‐5 (and other antimicrobial peptides). Alternatively, the presence of these bacteria may reflect their sustained transportation with food and saliva from the oral cavity. The lack of overt inflammation in the postoperative jejunal tissue suggests that HD‐5 (and also other Paneth cell‐derived products) is effective in inhibiting interactions between the bacteria and surface epithelial cells. However, the presence of luminal bacteria may cause malabsorption of nutrients in the proximal small intestine, which is likely to be exacerbated by the distal diversion of the liver and pancreatic secretions (fig 1).
In postoperative jejunal mucosal samples, there was an increase in HD‐5 expressing enterocytes and intermediate cells in the crypt. By contrast, there was no change in the number of villous intermediate cells. These latter cells were prominent in many jejunal sections both before and after surgery. They appear to have morphological and functional characteristics of both Paneth and goblet cells and we have previously shown in human ileal sections that intermediate cells express intestinal trefoil factor but not lysozyme.9 In the current study, we found that about 60% of intermediate cells in the jejunum express lysozyme, implying heterogeneity in these cells throughout the small intestine. The reasons for the prominence of jejunal intermediate cells in the population studied remain to be determined; one possibility being that factors associated with obesity act on intestinal epithelial stem cells. A direct role for obesity is unlikely as the number of villous intermediate cells did not change despite weight loss in the postoperative period. Comparative studies of small intestinal samples obtained from obese and non‐obese subjects could be of interest.
The finding of HD‐5‐expressing enterocytes in the crypt could be due either to the synthesis of HD‐5 by these cells or uptake of the defensin from the lumen. Since enterocyte HD‐5 immunoreactivity was localised to isolated enterocytes, we believe it more likely that these cells are synthesising the defensin. Limited availability of tissue samples has meant that it has not been possible to perform in situ hybridisation studies to determine whether HD‐5 immunoreactive enterocytes express the relevant transcripts. It is of interest that HD‐5‐immunoreactive epithelial cells that lack characteristic cytoplasmic granules of Paneth cells have recently been described in gastric intestinal metaplasia.23 Moreover, squamous and columnar epithelial cells of the human female reproductive tract have also been shown to express HD‐5.24 These studies provide support for the concept that epithelial cells other than Paneth cells may also express HD‐5.
Acknowledgements
We wish to thank Mr Tevor Gray for assistance in the immunoelectron microscopy studies.
Abbreviations
HD - human defensin
Footnotes
Funding: This work was supported in part by the Medical Research Council and the University of Nottingham.
Competing interests: None declared.
References
- 1.Ouellette A J, Bevins C L. Paneth cell defensins and innate immunity of the small bowel. Inflamm Bowel Dis 2001743–50. [DOI] [PubMed] [Google Scholar]
- 2.Cunliffe R N, Mahida Y R. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J Leukoc Biol 20047549–58. [DOI] [PubMed] [Google Scholar]
- 3.Erlandsen S L, Parsons J A, Taylor T D. Ultrastructural immunocytochemical localization of lysozyme in the Paneth cells of man. J Histochem Cytochem 197422401–413. [DOI] [PubMed] [Google Scholar]
- 4.Nevalainen T J, Gronroos J M, Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Invest 199572201–208. [PubMed] [Google Scholar]
- 5.Ouellette A J. Defensin‐mediated innate immunity in the small intestine. Best Pract Res Clin Gastroenterol 200418405–419. [DOI] [PubMed] [Google Scholar]
- 6.Elphick D A, Mahida Y R. Paneth cells: their role in innate immunity and inflammatory disease. Gut 2005541802–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mun E C, Blackburn G L, Matthews J B. Current status of medical and surgical therapy for obesity. Gastroenterology 2001120669–681. [DOI] [PubMed] [Google Scholar]
- 8.Sundbom M, Gustavsson S. Randomized clinical trial of hand‐assisted laparoscopic versus open Roux‐en‐Y gastric bypass for the treatment of morbid obesity. Br J Surg 200491418–423. [DOI] [PubMed] [Google Scholar]
- 9.Cunliffe R N, Rose F R, Keyte J.et al Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 200148176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ayabe T, Satchell D P, Wilson C L.et al Secretion of microbicidal alpha‐defensins by intestinal Paneth cells in response to bacteria. Nat Immunol 20001113–118. [DOI] [PubMed] [Google Scholar]
- 11.Tanabe H, Ayabe T, Bainbridge B.et al Mouse Paneth cell secretory responses to cell surface glycolipids of virulent and attenuated pathogenic bacteria. Infect Immun 2005732312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rumio C, Besusso D, Palazzo M.et al Degranulation of Paneth cells via toll‐like receptor 9. Am J Pathol 2004165373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lala S, Ogura Y, Osborne C.et al Crohn's disease and the NOD2 gene: a role for Paneth cells. Gastroenterology 200312547–57. [DOI] [PubMed] [Google Scholar]
- 14.Ogura Y, Lala S, Xin W.et al Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut 2003521591–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamal M, Wakelin D, Ouellette A J.et al Mucosal T cells regulate Paneth and intermediate cell numbers in the small intestine of T spiralis‐infected mice. Clin Exp Immunol 2001126117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshman E, Booth C, Potten C S. The intestinal epithelial stem cell. Bioessays 20022491–98. [DOI] [PubMed] [Google Scholar]
- 17.Porter E M, Van Dam E, Valore E V.et al Broad‐spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun 1997652396–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh D, Porter E, Shen B.et al Paneth cell trypsin is the processing enzyme for human defensin‐5. Nat Immunol 20023583–590. [DOI] [PubMed] [Google Scholar]
- 19.Ericksen B, Wu Z B, Lu W Y.et al Antibacterial activity and specificity of the six human alpha‐defensins. Antimicrob Agents Chemother 200549269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck C B, Day P M, Thompson C D.et al Human alpha‐defensins block papillomavirus infection. Proc Natl Acad Sci USA 20061031516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salzman N H, Chou M M, De Jong H.et al Enteric Salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect Immun 2003711109–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly P, Bajaj‐Elliott M, Katubulushi M.et al Reduced gene expression of intestinal alpha‐defensins predicts diarrhea in a cohort of African adults. J Infect Dis 20061931464–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen B, Porter E M, Reynoso E.et al Human defensin 5 expression in intestinal metaplasia of the upper gastrointestinal tract. J Clin Pathol 200558687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quayle A J, Porter E M, Nussbaum A A.et al Gene expression, immunolocalization, and secretion of human defensin‐5 in human female reproductive tract. Am J Pathol 19981521247–1258. [PMC free article] [PubMed] [Google Scholar]