Abstract
Aims
To determine whether basal‐like phenotype and vimentin and/or laminin are related in both sporadic/familial (BRCA1 or BRCA2 mutated) tumours.
Methods
230 non‐familial and 28 hereditary node‐negative invasive breast carcinomas were immunohistochemically analysed for oestrogen receptors (ER), progesterone receptors (PR), cytokeratin 5/6 (CK5/6), epidermal growth factor receptors (EGFR), Ki67, p53, vimentin and laminin, using tissue microarrays. Tumours were considered to have basal‐like phenotype if they were ER negative and HER2 negative, but positive for CK5/6 and/or EGFR.
Results
In sporadic tumours, vimentin expression was found in 77.8% cases with basal‐like phenotype and 15.5% of non‐basal cases (p<0.001). In familial cases, vimentin was expressed in 83.3% basal‐like cancers and 16.7% of non‐basal tumours (p<0.001). Vimentin expression was more frequent in BRCA1 than BRCA2 mutation carriers. Vimentin expressing tumours were associated with poor prognosis (p = 0.012) among patients not receiving adjuvant chemotherapy and showed a trend for local recurrence or visceral but not bone metastasis (p = 0.021). Laminin expression was also related to basal‐like phenotype in both sporadic/familial cases (p<0.001 and p = 0.007, respectively), but neither with prognosis nor recurrence pattern in sporadic cancers.
Conclusions
Vimentin and laminin expression is associated with basal‐like phenotype in breast cancer. Expression of vimentin and laminin is characteristic of BRCA1 associated tumours. Since vimentin and laminin staining is widely used by pathologists for diagnostic purposes, thus demonstrating the robustness of their specific antibodies, the immunohistochemical evaluation of these two molecules could be used in identification of basal‐like breast tumours in both sporadic/familial cases.
Keywords: vimentin, laminin, basal‐like phenotype, node‐negative breast carcinomas, immunohistochemistry
The existence of breast carcinomas that express basal keratins and other myoepithelial markers (basal‐like tumours) has been known for many years, but this tumour subtype has gained considerable notoriety due to its rediscovery by the use of cDNA microarrays.1,2,3 Moreover, recent evidence suggests that basal‐like phenotype might probably reflect a stem cell/progenitor phenotype rather than a myoepithelial differentiation.4 Several studies have indicated that basal‐like breast carcinomas are poorly differentiated, have specific morphological characteristics, a particular pattern of metastasis, and poor prognosis, although they might respond better to chemotherapy than do other breast cancer subtypes.5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 In addition, basal‐like carcinomas are remarkably frequent in BRCA1 mutation carriers.5,20,21,22,23,24,25,26,27 Nielsen et al have suggested that the use of a panel of four antibodies (ER, CK5/6, HER2, and EGFR) could accurately identify basal‐like tumours using standard available pathological tools.28 Based on their criteria we identified a subgroup of node‐negative breast carcinomas with poor prognosis if not treated with chemotherapy.29 In spite of these findings, basal‐like breast cancers remain a poorly characterised subgroup of tumours. The precise set of basal markers that defines this type of carcinoma remains to be established.
Vimentin is the most ubiquitous intermediate filament protein and is present in all primitive cell types, but during differentiation it is coexpressed or replaced by other intermediate filaments.30,31 In normal breast, vimentin is expressed by myoepithelial breast cells,32 and in breast cancer its expression is associated with high histological grade, lack of oestrogen receptor (ER) expression, epidermal growth factor receptor (EGFR) positivity and p53 expression.33,34,35,36,37,38,39,40 Recently, Korsching et al have shown that vimentin expression is associated with the expression of markers usually expressed by myoepithelial cells, such as CK5/6, smooth‐muscle actin, and CD10. However, these authors did not discuss whether vimentin‐positive breast carcinomas could be considered to be tumours with basal‐like phenotype.41,42 Very recently, Livasy et al studied the immunophenotype of a series of 56 breast tumours, including 18 cases that had been previously identified as basal‐like tumours based on cDNA expression profiling studies. They observed that vimentin was expressed more frequently in basal‐like tumours.43
The laminins are a family of glycoproteins that consist of three covalently linked chains, namely α, β, and γ. So far, 15 members of this family have been identified, the first 12 of which are the best studied. Laminins have been reported to play a role in the adhesion of cells to the matrix through various receptors that mostly belong to the family of integrin heterodimers. They are important in regulating cell migration, facilitating tumour invasion by favouring their attachment to the endothelial membrane of blood vessels. Although the expression of laminin in blood vessels and in the extracellular matrix component of breast cancer tissues has been studied, reports about its expression by neoplastic breast cancer cells are controversial.44,45,46,47,48 We have observed that laminin is expressed by myoepithelial cells surrounding ductal carcinoma in situ, suggesting that laminin could represent a novel marker of basal‐like phenotype.
The aim of this study was to test whether the expression of vimentin and/or laminin is associated with a basal‐like phenotype in a series of sporadic and familial node‐negative breast carcinomas.
Materials and methods
Tumour samples
This study involves both familial and sporadic (non‐familial) node‐negative breast carcinomas. The familial breast cancer group included 21 tumours from BRCA1‐mutation carriers and 18 tumours from BRCA2‐mutation carriers. The mutational study of the patients and some immunohistochemical and molecular characteristics of this group of tumours have been previously reported.21,24,29,49,50,51
We analysed a group of 230 sporadic invasive breast carcinomas diagnosed in the Hospital Universitario La Paz, Madrid, between 1988 and 2002. None of them met the criteria of familial cancer.50 Some clinicopathological characteristics of this series have been previously reported.29,51 All cases were primarily treated with surgery, consisting of segmental mastectomy with axillary node dissection or modified radical mastectomy according to the judgement of the multidisciplinary care team and patient preference. In addition, 97 patients (44.5%) received tamoxifen as adjuvant therapy, 43 (19.7%) were treated with tamoxifen and chemotherapy, and 71 (32.6%) patients received only chemotherapy.29 Chemotherapy regimen consisted of cyclophosphamide–methotrexate–5‐fluorouracil (CMF). The study was carried out with the approval of the Hospital La Paz ethical committee.
Tissue microarray construction
Representative areas from formalin‐fixed, paraffin‐embedded infiltrating carcinomas and 30 samples from non‐neoplastic breast tissue were carefully selected on H&E‐stained sections. Two 1 mm diameter tissue cores were obtained from each specimen. The tissue cores were precisely arrayed into a new paraffin block using a tissue microarray (TMA) workstation (Beecher Instruments, Silver Spring, MD, USA).
Immunohistochemistry
Immunohistochemical staining on TMA sections was performed by the EnVision method with a heat‐induced antigen‐retrieval step. Sections were immersed in boiling 10 mM sodium citrate at pH 6.5 for 2 min in a pressure cooker. Antibodies previously evaluated were ER, PR, HER2, Ki67, p53, and CK5/6.29 Mouse anti‐human laminin (LAM‐89, Novocastra) and vimentin (V9, Dako) monoclonal antibodies were applied at dilutions of 1:25 and 1:500, respectively. The robustness and sensitivity of these antibodies for immunohistochemistry has been previously demonstrated in several studies.32,33,34,35,36,37,38,39,40,41,42,52,53,54,55,56,57,58,59,60,61,62,63,64 Cases were considered positive for them, if at least 1% of invasive tumour cells had cytoplasmic and/or membranous staining. The same cut‐off point has been previously used by other authors.32,33,34,35,36,37,38,39,40,41,42,52,53,54,55,56,57,58,59,60,61,62,63,64 Moreover, since vimentin and laminin expression was heterogeneous within the tumour, a higher cut‐off value would probably produce a higher number of false negatives. Nonetheless using the 1% cut‐off we observed a frequency of vimentin positive expression (23%) which is similar to that reported in other series.32,33,34,35,36,37,38,39,40,41,42,52,53,54,55,56,57,58,59,60,61,62,63,64 As previously reported,29 a cut‐off of 1% was taken as the criterion for positivity for ER and PR, while more than 15% and 30% were the thresholds for being considered positive for Ki67 and p53, respectively. For HER2, only cases with 3+ membranous staining were scored as positive. Unequivocal membrane and cytoplasmic staining were considered as representing positivity for EGFR and CK5/6, respectively. Normal breast tissue was also evaluated as an internal control. In negative controls, the primary antibodies were omitted.
According to the criteria set out by Nielsen et al,28 those cases that were ER negative, HER2 negative, but positive for CK5/6 and/or EGFR were considered to belong to the basal‐like phenotype group.
Statistical analysis
To assess associations between categorical variables we used the χ2 contingency test or Fisher's exact test. Kaplan–Meier survival analyses were carried out for overall and breast cancer disease‐specific survival using the log‐rank test to examine differences between groups. Values of p<0.05 were considered significant. These analyses were carried out using SPSS V.12.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Results
Sporadic cases
In non‐neoplastic cells, vimentin was expressed in interstitial fibroblasts, endothelial cells, lymphoid cells, adipocytes and myoepithelial cells of normal mammary duct‐lobular units. Laminin was seen in the cytoplasm of some interstitial fibroblastic cells, adipocytes, and in the basal membrane surrounding vascular and normal mammary ducto‐alveolar structures. It was also seen in the cytoplasm of myoepithelial cells surrounding foci of the in situ carcinoma component (ductal carcinoma in situ).
Vimentin expression was found in 23.2% (52/224) of the breast carcinomas (fig 1 and table 1). When compared to other clinicopathological and immunohistochemical variables, vimentin positive expression was more frequently found in postmenopausal women, in tumours of greater size, of higher grade, and those that were CK5/6 and EGFR positive, ER and PR negative and showing higher proliferation index (Ki67) (table 1). Laminin expression was found in 17.6% (39/222) of the tumours studied (fig 1). Differences in the number of cores analysed for each marker were due to methodological circumstances which are intrinsic to the TMA technique. As table 1 shows, laminin expression was significantly more frequent in tumours that were of grade 3, with a high proliferation rate, CK5/6, EGFR and p53 positive, but ER and PR negative.
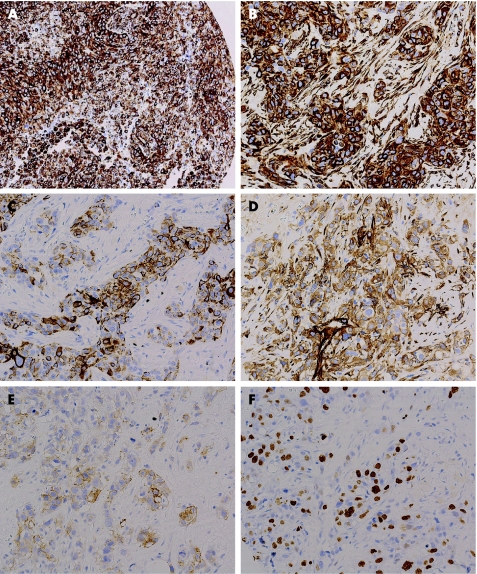
Figure 1 Expression of proteins studied by immunohistochemistry in tissue microarrays. (A) Vimentin immunoreactivity of a BRCA1‐related tumour. (B) Vimentin immunoreactivity of a sporadic breast carcinoma. (C) CK5 immunoreactivity in the same tumour depicted in B. (D) Laminin immunoreactivity of a sporadic breast carcinoma. (E) Epidermal growth factor receptor immunoreactivity in the same tumour depicted in D. (F) Ki67 immunoreactivity in the same tumour depicted in D and E. Original magnification, ×10 (A), ×20 (B–F).
Table 1 Relationships between vimentin and laminin expression and clinicopathological and immunohistochemical characteristics.
| Vimentin‐positive | p Value | Laminin‐positive | p Value | |
|---|---|---|---|---|
| Menopausal status | ||||
| Premenopausal | 17/99 (17.2%) | 17/98 (17.3%) | ||
| Postmenopausal | 34/116 (29.3%) | 0.037 | 22/115 (19.1%) | 0.737 |
| Size | ||||
| p T1 | 21/114 (18.4%) | 18/114 (15.8%) | ||
| p T2 | 23/70 (32.9%) | 0.026 | 17/69 (24.6%) | 0.141 |
| Grade | ||||
| 1 | 4/52 (7.7%) | 1/52 (1.9%) | ||
| 2 | 9/57 (15.8%) | 7/56 (12.5%) | ||
| 3 | 34/84 (40.5%) | <0.001 | 24/83 (28.9%) | <0.001 |
| ER | ||||
| Positive | 11/161 (6.8%) | 12/160 (7.5%) | ||
| Negative | 40/60 (66.7%) | <0.001 | 27/59 (45.8%) | <0.001 |
| PR | ||||
| Positive | 14/149 (9.4%) | 15/147 (10.2%) | ||
| Negative | 38/74 (51.4%) | <0.001 | 24/73 (32.9%) | <0.001 |
| p53 | ||||
| Positive | 16/60 (26.7%) | 17/59 (28.8%) | ||
| Negative | 35/157 (22.3%) | 0.497 | 22/156 (14.1%) | 0.012 |
| HER2 | ||||
| Positive | 6/37 (16.2%) | 8/35 (22.9%) | ||
| Negative | 46/187 (24.6%) | 0.270 | 31/187 (16.6%) | 0.370 |
| CK5/6 | ||||
| Positive | 20/35 (57.1%) | 12/35 (34.3%) | ||
| Negative | 32/186(17.2%) | <0.001 | 27/184 (14.7%) | 0.005 |
| EGFR | ||||
| Positive | 13/21 (61.9%) | 7/20 (35.0%) | ||
| Negative | 38/191 (19.9%) | <0.001 | 30/191 (15.7%) | 0.031 |
| Laminin | ||||
| Positive | 22/39 (56.4%) | |||
| Negative | 30/181 (16.6%) | <0.001 | ||
| Basal‐like* | ||||
| Positive | 21/27 (77.8%) | 11/26 (42.3%) | ||
| Negative | 30/194 (15.5%) | <0.001 | 28/193 (14.5%) | 0.001 |
EGFR, epidermal growth factor receptor; ER, oestrogen receptor; PR, progesterone receptor.
*According to Nielsen et al criteria.28
A basal‐like phenotype according to the criteria of Nielsen et al (ER negative, HER2 negative, but positive for CK5/6 and/or EGFR) was found in 27/227 tumours (11.9%). Of the cases with basal‐like phenotype, 77.8% expressed vimentin whereas 42.3% expressed laminin; thus positive expression of these markers was statistically associated with the basal‐like phenotype (table 1).
Vimentin and laminin expression and prognosis in sporadic N0 breast carcinomas
In this series, we have previously reported poor prognosis of basal‐like breast carcinomas that were not treated with chemotherapy (CMF).27 In addition, we observed that basal‐like tumours showed a greater tendency to metastasise to visceral rather than bone.
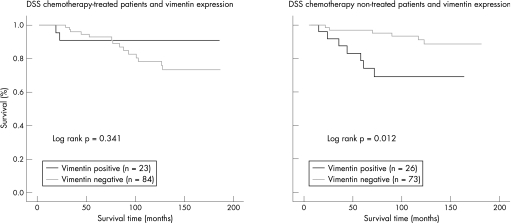
We examined whether vimentin and/or laminin expression was associated with prognosis or site of recurrence. Although laminin expression was not associated with these variables, vimentin was associated with poor prognosis in untreated patients (p = 0.012) (fig 2). Moreover, 1 of 47 (2.1%) vimentin‐expressing tumours metastasised to bone, while 3 (6.4%) and 6 (12.8%) suffered local recurrence or visceral metastases (mainly to lung), respectively (p = 0.021). Therefore, tumours showing vimentin expression were significantly more prone to visceral (lung) metastasis than were non‐vimentin‐expressing tumours (table 2).
Figure 2 Kaplan–Meier disease‐specific survival (DSS) curves for patients with breast cancer according to vimentin expression and treatment received.
Table 2 Relationship between laminin and vimentin expression and recurrence location.
| Recurrence | p Value* | ||||
|---|---|---|---|---|---|
| No recurrence | Local recurrence | Visceral metastases | Bone metastases | ||
| Vimentin | |||||
| Positive | 37/167 (22.2%) | 3/13 (23.1%) | 6/10 (60%) | 1/14 (7.1%) | |
| Negative | 130/167 (77.8%) | 10/13 (76.9%) | 4/10 (40.0%) | 13/14 (92.9%) | 0.021 |
| Laminin | |||||
| Positive | 33/166 (19.9%) | 2/13 (15.4%) | 2/9 (22.2%) | 1/14 (7.1%) | |
| Negative | 133/166 (80.1%) | 11/13 (84.6%) | 7/9 (77.8%) | 13/14 (92.9%) | 0.673 |
*p value of χ2 test.
Familial cases
Vimentin expression was more prevalent among BRCA1 (84.6%, 11/13 cases) than among BRCA2 carcinomas (15.4%, 2/13 cases) (p = 0.037). Laminin expression was also more frequent in BRCA1 (80%, 12/15 cases) than in BRCA2 carcinomas (20.0%, 3/15 cases), although such a difference in proportions is not statistically significant (p = 0.081) for a sample of this size.
Sixteen of 34 familial cases (47.1%) had basal‐like phenotype according to the criteria of Nielsen et al. This phenotype predominated among BRCA1 tumours (87.5%, 14/16 cases), but was infrequent among BRCA2 carcinomas (12.5%, 2/16); this difference was statistically significant (p = 0.004). Vimentin was expressed in 83.3% (10/12 cases) of basal‐like tumours, but in only 16.7% (2/12) of non basal‐like cases (p<0.001). Moreover, 11/15 laminin‐positive cases (73.3%) had basal‐like phenotype according to the criteria of Nielsen et al, while 26.7% (4/15) of the cases did not. This association is statistically significant (p = 0.007).
Discussion
Several reports have established that breast carcinomas with expression of basal markers show specific morphological, proliferative and prognostic characteristics.3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 This group of tumours seems to be well identified by the use of cDNA arrays, but its recognition with immunohistochemical markers is less well established.2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17 By studying immunohistochemical markers in breast carcinomas previously evaluated by cDNA arrays, Perou's group proposed a panel of four antibodies (ER, HER2, CK5/6, and EGFR) to identify breast carcinomas with a basal‐like phenotype.28 This group subsequently found that 17/18 tumours with a basal‐like phenotype determined by cDNA microarrays expressed vimentin.43 To test the hypothesis that vimentin expression is associated with the basal‐like phenotype, we analysed a group of node‐negative breast carcinomas in which this phenotype has been characterised according to the criteria of Nielsen et al.28
In the present study we found a direct statistically significant association between vimentin expression and greater size, higher grade and proliferation index, and ER and PR negativity, thus corroborating previous reports of vimentin expression in breast carcinomas.33,34,35,36,37,38,39,40 In addition, vimentin was associated with CK5/6 and EGFR expression and for this reason was also associated with basal‐like phenotype. In this sense, nearly 80% of breast carcinomas with basal‐like phenotype expressed vimentin, but only 15% of non‐basal tumours did. These results and those recently reported by Livasy et al suggest that vimentin could be an excellent marker for defining the basal phenotype immunohistochemically43 (in our series it had a specificity of 85% and sensitivity of 78%).
Vimentin expression in breast cancer cells in culture has often been associated with an increase in invasive/migratory potential,30,51,53,55 although its significance in vivo is controversial. Although many studies have not detected any relationship between vimentin expression and survival and/or recurrence,55,58,59,61 others have found that vimentin is an indicator of poor prognosis.36,37,56,57,62,63 These evident discrepancies may be due to the methodological variations among the studies, such as differences in immunohistochemical evaluation, and in the characteristics of the tumours analysed. However, we did not find any evident relationship between variations in the immunohistochemical methods and the different results. Nevertheless, it is more likely that the observed discrepancies may be probably due to the differences in clinicopathological characteristics of the tumours analysed. For instance, it is worth noting that the prognostic value of vimentin expression may be influenced by the lymph node status. Vimentin expression has been associated with poor prognosis only in lymph node negative cases,37,56,63 but not in series containing node positive and negative tumours,59,61 or when lymph node status was unknown.55 However, in the present series, comprising 230 node‐negative cases, vimentin expression was not related to prognosis considering all tumours, but was significantly correlated with poor prognosis in the postmenopausal subgroup of patients (considering both treated and non‐chemotherapy treated patients; p = 0.006, data not shown) and when only the patients not receiving postoperative chemotherapy were selected (fig 2). In agreement with our results, in the series studied by Russo et al,58 in which 86% of the patients were postmenopausal, and by Thomas et al36 (all cases postmenopausal), a correlation was found between vimentin positivity and higher probability of early relapse. Moreover, some histopathological subtypes of breast cancers have been hardly related to better prognosis, as with typical and atypical medullary carcinomas,64,65 which constituted most of the series studied by Holck et al.56 Furthermore, regarding chemotherapy, it is worth noting that in most of the studies reporting an association of vimentin with prognosis, most of the studied patients had not received chemotherapy.56,57,63 Together these data suggest that vimentin positivity may have prognostic value only in specific subgroups of breast cancer patients, probably in those postmenopausal, node‐negative patients who have not received chemotherapy.
Additionally, consistent with two previous studies,37,56,62 we found that vimentin‐positive tumours showed a preference for visceral rather than bone metastasis. Similarly, we observed a higher frequency of visceral metastasis in basal‐like compared to non‐basal tumours in the present series.29
However, other markers associated with a basal‐like phenotype, such as CK5/6, fascin,29 caveolin 151 or laminin (see below) did not show these associations with prognosis or recurrence pattern, suggesting that vimentin expression is related to the biological behaviour of basal‐like carcinomas, although further studies with larger series of basal‐like carcinomas are required. In addition, its presence might make it possible to treat these tumours with tyrosine kinase inhibitors, since Lim et al reported that cytoskeletal proteins including vimentin are one of the most abundant tyrosine phosphoproteins in breast tumours.67
Laminin cytoplasmic expression in breast cancer has rarely been reported and its presence is thought to represent a potential for neoplastic cells to synthesise this molecule. The percentage of laminin‐positive cases varies among series, ranging from none to all cases studied.68,69,70,71,72,73,74 Although most studies have not revealed any association between laminin expression and clinicopathological features, De Iorio et al72 found a direct significant association between laminin expression and tumour grade, ER positivity, number of mitoses, lymph node metastasis, DNA non‐diploid content and recurrence, while Vesaturo et al74 found an inverse relationship between laminin and Ki67 expression. We found laminin immunoreactivity in 17.9% of sporadic breast carcinomas. Moreover, its expression was related to Ki67, p53, CK5/6, and EGFR expression, and to ER and PR negativity, and therefore it was frequently (42.3%) expressed in cases with basal‐like phenotype. The relationship between laminin expression and prognosis is also controversial. De Iorio et al found a significant association between laminin expression and lymph node metastasis and recurrence.72 Molino et al found that, in the absence of nodal involvement, the risk of relapse was higher in patients who were laminin‐positive than in those who were laminin‐negative; in the case of patients with four or more involved nodes, the prognostic role of laminin was reversed.73 In contrast, most studies did not find any association between laminin immunoreactivity and prognosis. As commented before, differences among the findings of studies, including the present one, probably arise from methodological variations (immunohistochemical evaluation and/or tumour selection). In the present series, no association between laminin expression and prognosis was found. Our data suggest that although laminin expression was related to the basal‐like phenotype tumours, its possible contribution to their aggressive behaviour should be analysed in an larger series of cases.
Take‐home messages
Vimentin and laminin expression are associated with basal‐like phenotype (tumours ER negative, HER2 negative, but positive for CK5/6 and/or EGFR) in both sporadic and familial breast carcinomas.
In familial breast cancer, vimentin and laminin expression is significantly more frequent in BRCA1 than BRCA2 mutation carriers.
Vimentin but not laminin staining is associated with poor prognosis among patients not receiving adjuvant chemotherapy.
Vimentin‐positive tumours show a preference for visceral rather than bone metastasis.
Furthermore, vimentin and laminin expression have not been analysed in hereditary breast cancer before. In the current study, these markers were expressed mainly in hereditary cancers with a basal‐like phenotype, for which reason they were common among BRCA1‐associated carcinomas. Vimentin and laminin expression were found in more than 80% of BRCA1‐associated breast carcinomas, a frequency higher than that observed with other basal markers such as CK5/6.25,27 Therefore, as has been proposed for CK5/6,20 we suggest that these markers might be useful for the more accurate prediction of the probability of carrying a BRCA1 mutation, although further studies are needed to evaluate this possibility.
In conclusion, this study shows that the expression of laminin and, in particular, vimentin is associated with a basal‐like phenotype in breast cancer. In addition, the current study provides the first report of vimentin and laminin expression in hereditary breast cancer, these being characteristics of BRCA1‐associated tumours.
Abbreviations
EGFR - epidermal growth factor receptor
ER - oestrogen receptor
PR - progesterone receptor
Footnotes
Funding: This study was supported by the Spanish Ministry of Education and Science (SAF2004‐08258‐C02‐01) and the Instituto de Salud Carlos III (PI051890). Socorro María Rodríguez‐Pinilla is the recipient of a research grant from the Fondo de Investigación Sanitaria, Spain. Emiliano Honrado is funded by the Foundation of the Spanish Association against Cancer (AECC). Gema Moreno Bueno is a junior investigator of the Ramón y Cajal Programme (2004).
Competing interests: None declared.
References
- 1.Santini D, Ceccarelli C, Taffurelli M.et al Differentiation pathways in primary invasive breast carcinoma as suggested by intermediate filament and biopathological marker expression. J Pathol 1996179386–391. [DOI] [PubMed] [Google Scholar]
- 2.Gusterson B A, Ross D T, Heath V J.et al Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res 20057143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou C M, Sorlie T, Eisen M B.et al Molecular portraits of human breast tumours. Nature 2000406747–752. [DOI] [PubMed] [Google Scholar]
- 4.Abd El‐Rehim D M, Pinder S E, Paish C E.et al Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol 2004203661–671. [DOI] [PubMed] [Google Scholar]
- 5.Asselin‐Labat M L, Shackleton M, Stingl J.et al Steroid hormone receptor status of mouse mammary stem cells. J Natl Cancer Inst 2006981011–1014. [DOI] [PubMed] [Google Scholar]
- 6.Sorlie T, Tibshirani R, Parker J.et al Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 20031008414–8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van't Veer L J, Dai H, van de Vijver M J.et al Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002415530–535. [DOI] [PubMed] [Google Scholar]
- 8.Van de Rijn M, Perou C M, Tibshirani R.et al Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol 20031631991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West M, Blanchette C, Dressman H.et al Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci USA 20019811462–11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makretsov N A, Huntsman D G, Nielsen T O.et al Hierarchical clustering analysis of tissue microarray immunostaining data identifies prognostically significant groups of breast carcinoma. Clin Cancer Res 2004106143–6151. [DOI] [PubMed] [Google Scholar]
- 11.Jones C, Ford E, Gillett C.et al Molecular cytogenetic identification of subgroups of grade III invasive ductal breast carcinomas with different clinical outcomes. Clin Cancer Res 2004105988–5997. [DOI] [PubMed] [Google Scholar]
- 12.Jones C, Nonni A V, Fulford L.et al CGH analysis of ductal carcinoma of the breast with basaloid/myoepithelial cell differentiation. Br J Cancer 200185422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsuda H, Takarabe T, Hasegawa T.et al Myoepithelial differentiation in high‐grade invasive ductal carcinomas with large central acellular zones. Hum Pathol 1999301134–1139. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda H, Takarabe T, Hasegawa F.et al Large, central acellular zones indicating myoepithelial tumor differentiation in high‐grade invasive ductal carcinomas as markers of predisposition to lung and brain metastases. Am J Surg Pathol 200024197–202. [DOI] [PubMed] [Google Scholar]
- 15.Jacquemier J, Padovani L, Rabayrol L.et al Typical medullary breast carcinomas have a basal/myoepithelial phenotype. J Pathol 2005207260–268. [DOI] [PubMed] [Google Scholar]
- 16.Minn A J, Gupta G P, Siegel P M.et al Genes that mediate breast cancer metastasis to lung. Nature 2005436518–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouzier N, Perou C M, Symmans W F.et al Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005115678–5685. [DOI] [PubMed] [Google Scholar]
- 18.Fulford L G, Easton D F, Reis‐Filho J S.et al Specific morphological features predictive for basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathology 20064922–34. [DOI] [PubMed] [Google Scholar]
- 19.Reis‐Filho J S, Milanezi F, Steele D.et al Metaplastic breast carcinomas are basal‐like tumors. Histopathology 20064910–21. [DOI] [PubMed] [Google Scholar]
- 20.Foulkes W D, Brunet J S, Stefansson I M.et al The prognostic implication of the basal‐like (cyclin‐E high/p27 low/p53+/glomeruloid‐microvascular‐proliferation+) phenotype of BRCA1‐related breast cancers. Cancer Res 200464830–835. [DOI] [PubMed] [Google Scholar]
- 21.Palacios J, Honrado E, Osorio A.et al Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res Treat 2005905–14. [DOI] [PubMed] [Google Scholar]
- 22.Foulkes W D, Stefansson I M, Chappuis P O.et al Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 2003951482–1485. [DOI] [PubMed] [Google Scholar]
- 23.van der Groep P, Bouter A, van der Zanden R.et al Re: Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 200496712–713. [DOI] [PubMed] [Google Scholar]
- 24.Palacios J, Honrado E, Osorio A.et al Re: Germline mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 200496712–714. [DOI] [PubMed] [Google Scholar]
- 25.Laakso M, Loman N, Borg A.et al Cytokeratin 5/14‐positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod Pathol 2005181321–1328. [DOI] [PubMed] [Google Scholar]
- 26.Arnes J B, Brunet J S, Stefansson I.et al Placental cadherin and the basal epithelial phenotype of BRCA1‐related breast cancer. Clin Cancer Res 2005114003–4011. [DOI] [PubMed] [Google Scholar]
- 27.Lakhani S R, Reis‐Filho J S, Fulford L.et al Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res 2005115175–5180. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen T O, Hsu F D, Jensen K.et al Immunohistochemical and clinical characterization of the basal‐like subtype of invasive breast carcinoma. Clin Cancer Res 2004105367–5374. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez‐Pinilla S. M, Sarrió D, Honrado E, et al. Prognostic significance of basal‐like phenotype and fascin expression in node‐negative invasive breast carcinomas. Clin Cancer Res 2006121533–1539. [DOI] [PubMed] [Google Scholar]
- 30.Hendrix M J, Seftor E A, Seftor R E.et al Experimental co‐expression of vimentin and keratin intermediate filaments in human breast cancer cells results in phenotypic interconversion and increased invasive behaviour. Am J Pathol 1997150483–495. [PMC free article] [PubMed] [Google Scholar]
- 31.Dandachi N, Hauser‐Kronberger C, More E.et al Co‐expression of tenascin‐C and vimentin in human breast cancer cells indicates phenotypic transdifferentiation during tumour progression: correlation with histopathological parameters, hormone receptors, and oncoproteins. J Pathol 2001193181–189. [DOI] [PubMed] [Google Scholar]
- 32.Guelstein V I, Tchypysheva T A, Ermilova V D.et al Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer 198842147–153. [DOI] [PubMed] [Google Scholar]
- 33.Santini D, Ceccarelli C, Taffurelli M.et al Differentiation pathways in primary invasive breast carcinoma as suggested by intermediate filament and biopathological marker expression. J Pathol 1996179386–391. [DOI] [PubMed] [Google Scholar]
- 34.Raymond W A, Leong A S. Vimentin—a new prognostic marker in breast carcinoma? J Pathol 1989158107–114. [DOI] [PubMed] [Google Scholar]
- 35.Domagala W, Lasota J, Bartkowiak J.et al Vimentin is preferentially expressed in human breast carcinomas with low estrogen receptor and high Ki67 growth fraction. Am J Pathol 1990136219–227. [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas P A, Kirschmann D A, Cerhan J R.et al Association between keratin and vimentin expression, malignant phenotype, and survival in postmenopausal breast cancer patients. Clin Cancer Res 199952698–2703. [PubMed] [Google Scholar]
- 37.Domagala W, Lasote J, Dukowicz A.et al Vimentin expression appears to be associated with poor prognosis in node‐negative ductal NOS breast carcinomas. Am J Pathol 19901371299–1304. [PMC free article] [PubMed] [Google Scholar]
- 38.Kontselini H, Markopoulos C, Lambropoulouet al Relationship of epidermal growth factor receptor (EGFR), proliferating cell nuclear antigen (PCNA) and vimentin expression and various prognostic factors in breast cancer patients. Cytopathology 1995614–21. [DOI] [PubMed] [Google Scholar]
- 39.Umemura S, Takekoshi S, Suzuki Y.et al Estrogen receptor‐negative and human epidermal growth factor receptor 2‐negative breast cancer tissue have the highest ki‐67 labeling index and EGFR expression: gene amplification does not contribute to EGFR expression. Oncol Rep 200514337–343. [PubMed] [Google Scholar]
- 40.Tsuda H, Morita D, Kimura M.et al Correlation of KIT and EGFR overexpression with invasive ductal breast carcinoma of the solid‐tubular subtype, nuclear grade 3, and mesenchymal or myoepithelial differentiation. Cancer Sci 20059648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korsching E, Packeisen J, Liedtke C.et al The origin of vimentin expression in invasive breast cancer: epithelial‐mesenchymal transition, myoepithelial histogenesis from progenitor cells with bilinear differentiation potential? J Pathol 2005206451–457. [DOI] [PubMed] [Google Scholar]
- 42.Reis‐Filho J S. Re: Korsching, et al. The origin of vimentin expression in invasive breast cancer: epithelial‐mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential, J Pathol 2005206451–457. [DOI] [PubMed] [Google Scholar]
- 43.Livasy C A, Karaca G, Nanda R.et al Phenotypic evaluation of the basal‐like subtype of invasive breast carcinoma. Modern Pathol 200619264–271. [DOI] [PubMed] [Google Scholar]
- 44.Engbring J A, Kleinman H K. The basement membrane matrix in malignancy. J Pathol 2003200465–470. [DOI] [PubMed] [Google Scholar]
- 45.Määttä M, Virtanen I, Burgeson R.et al Comparative analysis of the distribution of laminin chains in the basement membranes in some malignant epithelial tumors: the α1 chain of laminin shows a selected expression pattern in human carcinomas. J Histochem Cytochem 200149711–725. [DOI] [PubMed] [Google Scholar]
- 46.Holler E. Laminin isoform expression in breast tumors. Brest Cancer Res 20057166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gudjonsson T, Rønnov‐Jessen L, Villadsen R.et al Normal and tumor‐derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 200211539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adriance M C, Inman J L, Petersen O W.et al Myoepithelial cells: good fences make good neighbors. Breast Cancer Res 20057190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honrado E, Benitez J, Palacios J. The molecular pathology of hereditary breast cancer: genetic testing and therapeutics implications. Mod Pathol 2005181305–1320. [DOI] [PubMed] [Google Scholar]
- 50.Palacios J, Honrado E, Osorio A.et al Immunohistochemical characteristics defined by tissue‐microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations: differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin Cancer Res 200313606–3614. [PubMed] [Google Scholar]
- 51.Rodríguez‐Pinilla S M, Honrado E, Hardisson D.et al Caveolin‐1 expression is associated with a basal‐like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat 20069985–90. [DOI] [PubMed] [Google Scholar]
- 52.Thompson E W, Paik S, Brunner N.et al Association of increased basement membrane invasiveness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol 1992150534–544. [DOI] [PubMed] [Google Scholar]
- 53.Hendrix M J, Seftor E A, Chu Y W.et al Role of intermediate filaments in migration, invasion and metastasis. Cancer Metastasis Rev 199615507–525. [DOI] [PubMed] [Google Scholar]
- 54.Zajchowski D A, Bartholdi M F, Gong Y.et al Identification of gene expression profiles that predict the aggressive behaviour of breast cancer cells. Cancer Res 2001615168–5178. [PubMed] [Google Scholar]
- 55.Willipinski‐Stapelfeldt B, Riethdorf S, Assmann V.et al Changes in cytoskeletal protein composition indicative of an epithelial‐mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res 2005118006–8014. [DOI] [PubMed] [Google Scholar]
- 56.Holck S, Pedersen L, Schiodt T.et al Vimentin expression in 98 breast cancers with medullary features and its prognostic significance. Virchows Arch A Pathol Anat Histopathol 1993422475–479. [DOI] [PubMed] [Google Scholar]
- 57.Domagala W, Striker G, Szadowska A.et al P53 protein and vimentin in invasive ductal NOS breast carcinoma‐relationship with survival and sites of metastases. Eur J Cancer 1994301527–1534. [DOI] [PubMed] [Google Scholar]
- 58.Russo A, Bazan V, Morello V.et al Vimentin expression, proliferating cell nuclear antigen and flow cytometric factors. Prognostic role in breast cancer. Anal Quant Cytol Histol 199416365–374. [PubMed] [Google Scholar]
- 59.Seshadri R, Raymond W A, Leong A S.et al Vimentin expression is not associated with poor prognosis in breast cancer. Int J Cancer 199667353–356. [DOI] [PubMed] [Google Scholar]
- 60.Bozcuk H, Uslu G, Pestereli E.et al Predictors of distant metastasis at presentation in breast cancer: a study also evaluating associations among common biological indicators. Breast Cancer Res Treat 200168239–248. [DOI] [PubMed] [Google Scholar]
- 61.Scawn R, Shousha S. Morphologic spectrum of estrogen receptor negative breast carcinoma. Arch Pathol Lab Med 2002126325–330. [DOI] [PubMed] [Google Scholar]
- 62.Heatley M K, Ewing P, Odling Smee W.et al Vimentin expression does not assist in predicting survival in ductal carcinoma of the breast. Pathology 200234230–232. [DOI] [PubMed] [Google Scholar]
- 63.Niveditha S R, Bajaj P. Vimentin expression in breast carcinomas. Indian J Pathol Microbiol 200346579–584. [PubMed] [Google Scholar]
- 64.Tomasino R M, Russo A, Bazan V.et al Evaluation of integrated morpho‐biological indicators in breast cancer. In Vivo 19937601–607. [PubMed] [Google Scholar]
- 65.Allemani C, Sant M, Berrino F.et al Prognostic value of morphology and hormone receptor status in breast cancer—a population‐based study. Br J Cancer 2004911263–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Page D L. Special types of invasive breast cancer, with clinical implications. Am J Surg Pathol 200327832–835. [DOI] [PubMed] [Google Scholar]
- 67.Lim Y P, Wong Ch Y, Ooi L L.et al Selective tyrosine hyperphosphorylation of cytoskeletal and stress proteins in primary human breast cancers: implications for adjuvant use of kinase‐inhibitory drugs. Clin Cancer Res 2004103980–3987. [DOI] [PubMed] [Google Scholar]
- 68.Gorczyca W, Holm R, Nesland J M. Laminin production and fibronectin immunoreactivity in breast carcinomas. Anticancer Res 199313851–858. [PubMed] [Google Scholar]
- 69.Rudland P S, Leinster S J, Winstanley J.et al Immunocytochemical identification of cell types in benign and malignant breast diseases: variations in cell markers accompany the malignant state. J Histochem Cytochem 199341543–553. [DOI] [PubMed] [Google Scholar]
- 70.Pellegrini R, Martignone S, Tagliabue E.et al Prognostic significance of laminin production in relation with its receptor expression in human breast carcinomas. Breast Cancer Res Treatment 199535195–199. [DOI] [PubMed] [Google Scholar]
- 71.Tagliabue E, Ghirelli C, Squicciarini P.et al Prognostic value of α6β4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res 19984407–410. [PubMed] [Google Scholar]
- 72.De Iorio P, Midulla C, Pisani T.et al Implication of laminin and collagen type IV expression in the expression of breast carcinoma. Anticancer Res 2001211395–1399. [PubMed] [Google Scholar]
- 73.Molino A, Pedersini R, Micciolo R.et al Prognostic significance of laminin, laminin receptor, and bone marrow micrometastases in breast cancer patients. Are these markers of aggressive behaviour and metastatic potential? Appl Immunohistochem Mol Morphol 200311311–318. [DOI] [PubMed] [Google Scholar]
- 74.Vesaturo F, Sallusti E, Gradilone A.et al Comparison of extracellular matrix and apoptotic markers between benign lesions and carcinomas in human breast. Int J Oncol 2005271005–1011. [PubMed] [Google Scholar]