Abstract
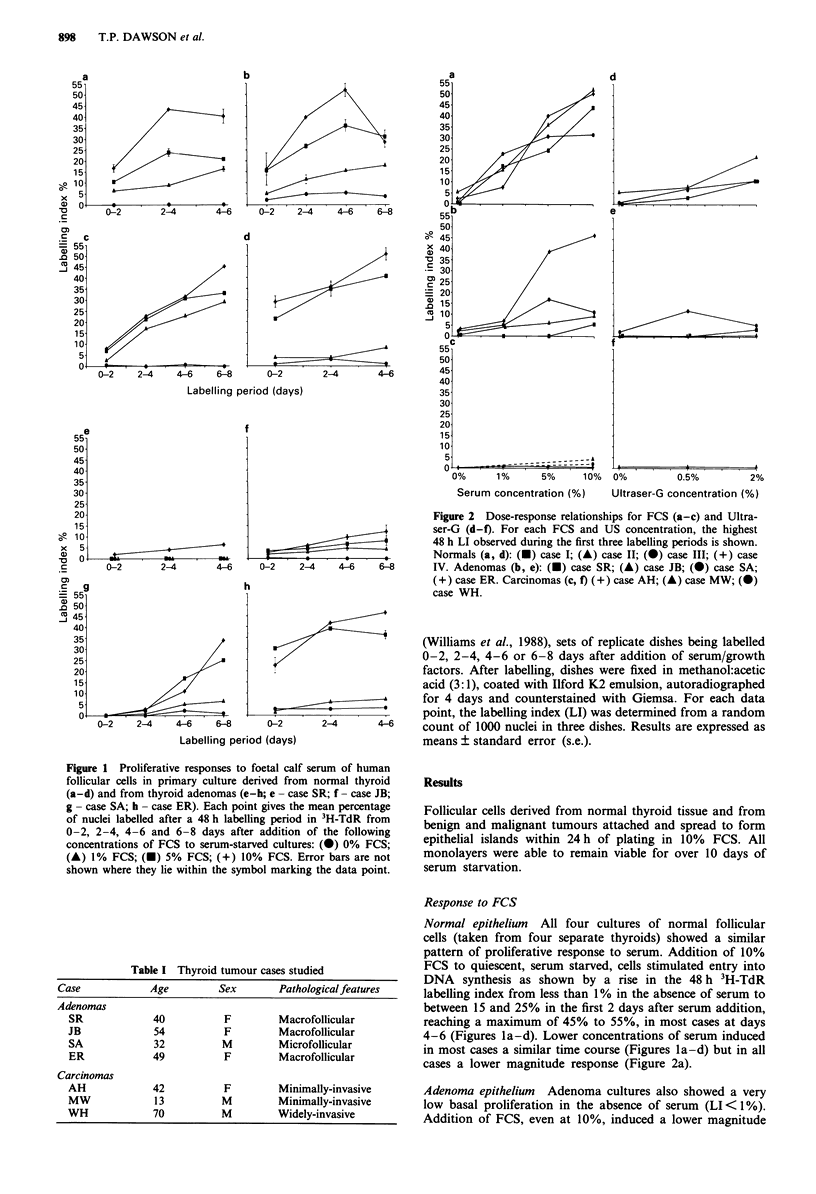
We have examined the proliferative response (DNA synthesis) of primary thyroid epithelial cultures to serum and a defined serum-substitute. These cultures were derived from normal human thyroid and from thyroid adenomas and carcinomas. All normal cultures showed a dose-dependent response, with a maximum 3H-thymidine labelling index of around 50%. Three out of the four adenomas demonstrated a much reduced or delayed response under the same conditions. In two carcinomas, labelling was never more than 5% and in one case was undetectable. This inverse relationship between the degree of in vivo malignancy and proliferative response in vitro has important implications for the interpretation of tissue culture models of epithelial neoplasia and also offers the potential for isolating novel growth factors specific for thyroid cancer cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartek J., Iggo R., Gannon J., Lane D. P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene. 1990 Jun;5(6):893–899. [PubMed] [Google Scholar]
- Buehring G. C., Williams R. R. Growth rates of normal and abnormal human mammary epithelia in cell culture. Cancer Res. 1976 Oct;36(10):3742–3747. [PubMed] [Google Scholar]
- Kirkland W. L., Yang N. S., Jorgensen T., Longley C., Furmanski P. Growth of normal and malignant human mammary epithelial cells in culture. J Natl Cancer Inst. 1979 Jul;63(1):29–41. [PubMed] [Google Scholar]
- Lemoine N. R., Mayall E. S., Wyllie F. S., Williams E. D., Goyns M., Stringer B., Wynford-Thomas D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene. 1989 Feb;4(2):159–164. [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Paraskeva C., Buckle B. G., Sheer D., Wigley C. B. The isolation and characterization of colorectal epithelial cell lines at different stages in malignant transformation from familial polyposis coli patients. Int J Cancer. 1984 Jul 15;34(1):49–56. doi: 10.1002/ijc.2910340109. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Thompson N. L., Heine U., Flanders C., Sporn M. B. Transforming growth factor-beta: possible roles in carcinogenesis. Br J Cancer. 1988 Jun;57(6):594–600. doi: 10.1038/bjc.1988.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas-Morvan C., Talbot M., Chambard M. Culture en gel de collagène de cancers thyroïdiens humains. C R Seances Acad Sci III. 1983;296(9):441–448. [PubMed] [Google Scholar]
- Williams D. W., Williams E. D., Wynford-Thomas D. Evidence for autocrine production of IGF-1 in human thyroid adenomas. Mol Cell Endocrinol. 1989 Jan;61(1):139–143. doi: 10.1016/0303-7207(89)90199-8. [DOI] [PubMed] [Google Scholar]
- Williams D. W., Williams E. D., Wynford-Thomas D. Loss of dependence on IGF-1 for proliferation of human thyroid adenoma cells. Br J Cancer. 1988 Jun;57(6):535–539. doi: 10.1038/bjc.1988.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. W., Wynford-Thomas D., Williams E. D. Control of human thyroid follicular cell proliferation in suspension and monolayer culture. Mol Cell Endocrinol. 1987 May;51(1-2):33–40. doi: 10.1016/0303-7207(87)90116-x. [DOI] [PubMed] [Google Scholar]


