Abstract
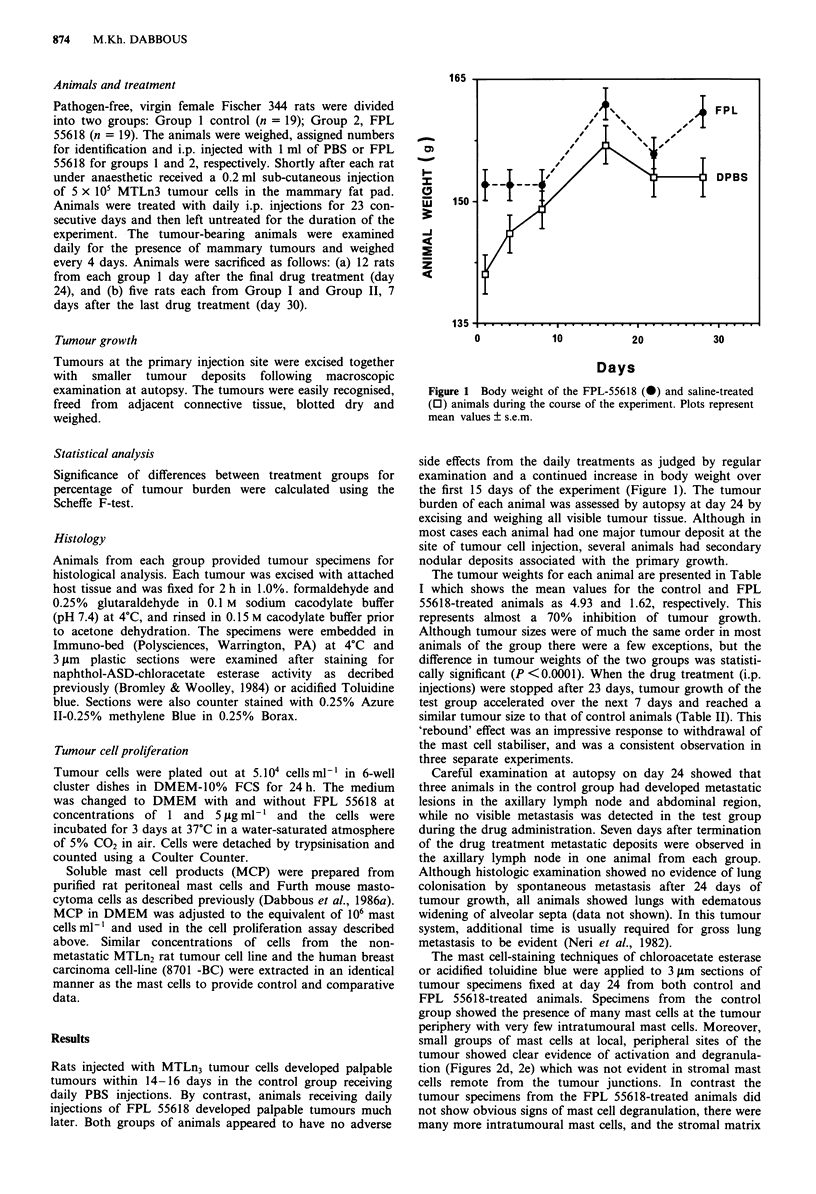
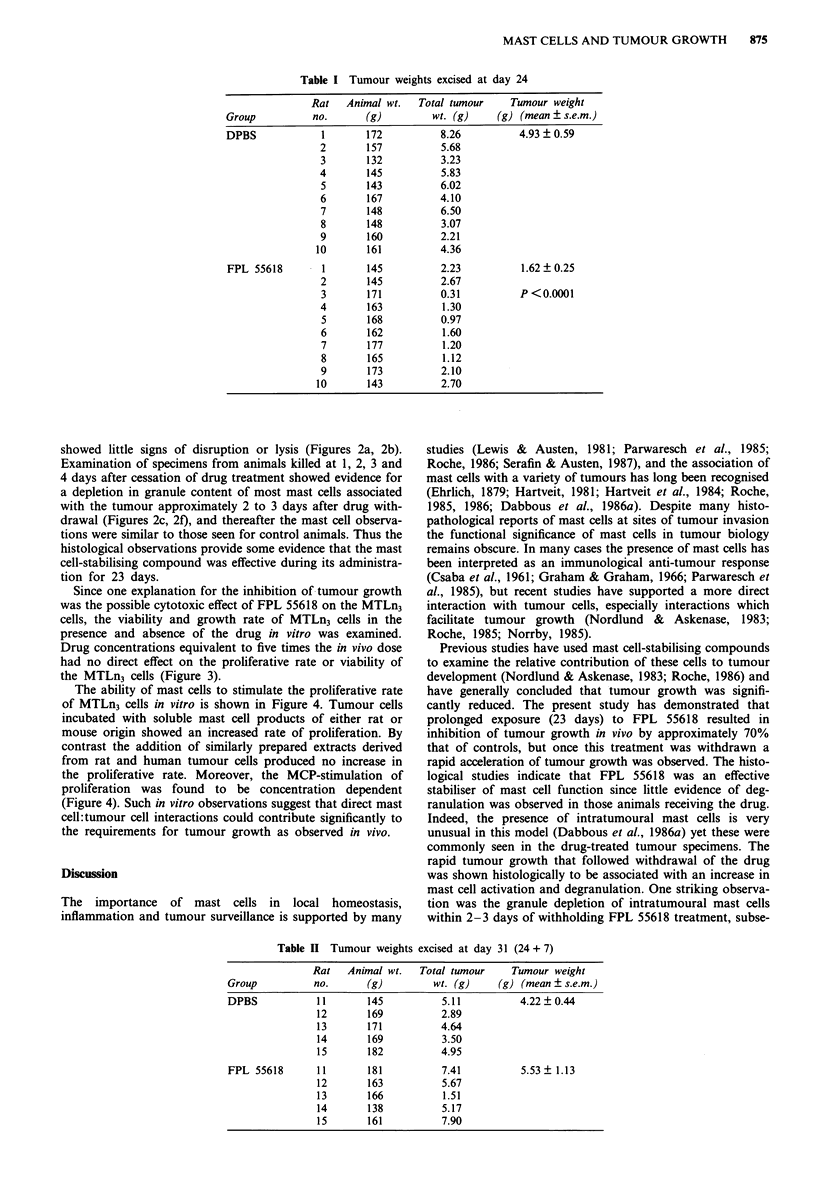
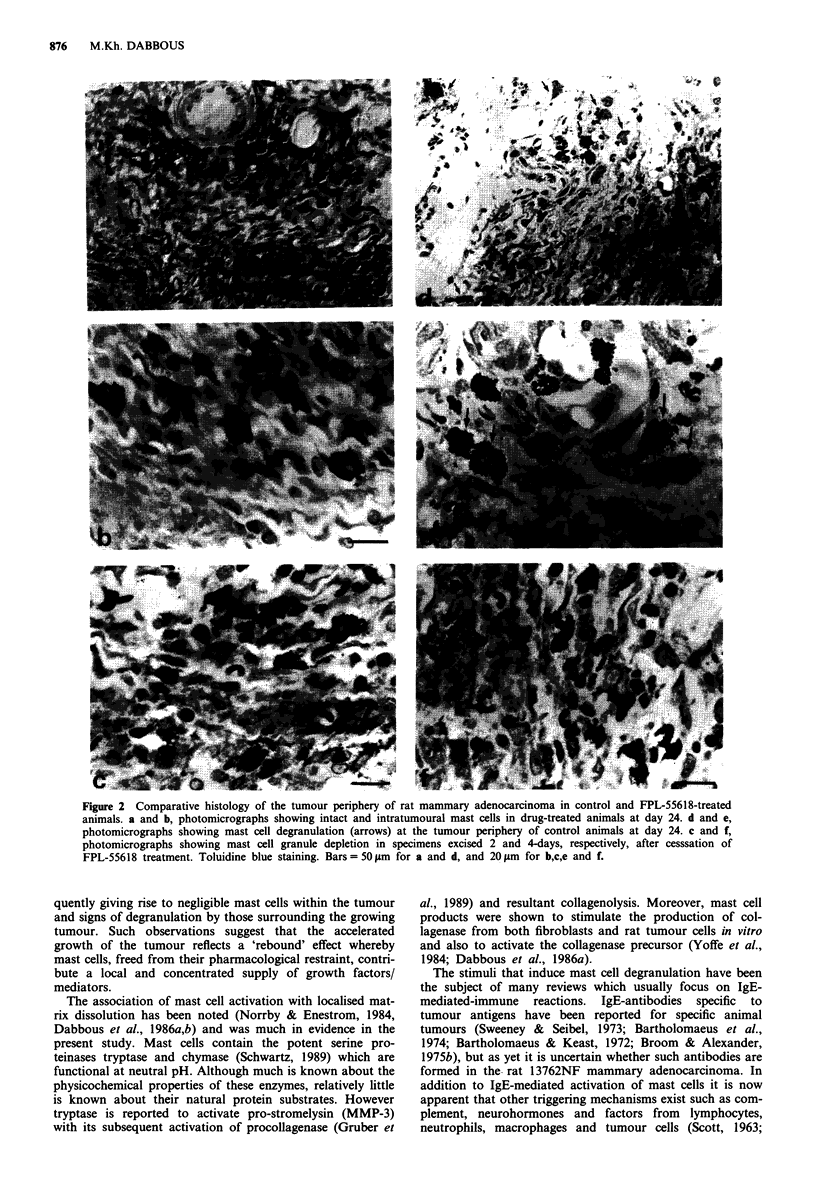
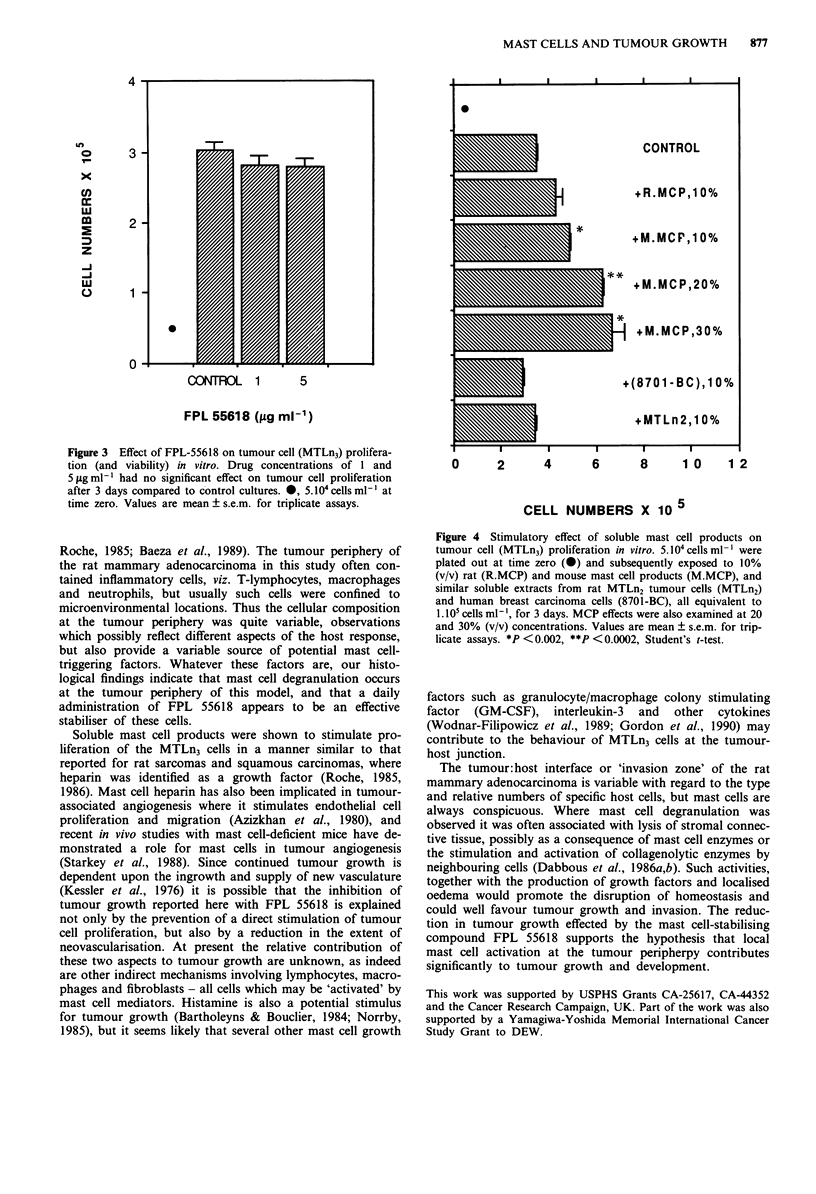
Mast cells were shown to accumulate around the periphery of the invasive and metastatic rat mammary adenocarcinoma (MTLn3), and histological evidence of mast cell degranulation was observed during the later stages of this model. To assess the physiological role of mast cells in vivo we have used the mast cell-stabilising compound FPL 55618 applied i.p. daily at 1 mg kg-1 for 23 days. Using groups of 12 rats we have found that this compound inhibited tumour growth at the primary site by as much as 70% in most of the treated animals compared with the control group which received equivalent volumes of saline. When the drug treatment was stopped after 23 days, tumour growth of the test group accelerated over the next 7 days and reached a similar tumour size to that of control animals. Histological studies of the tumour and contiguous host tissue at day 24 of the experiment revealed numerous extra-tumoural mast cells often showing signs of degranulation at several sites around the tumour periphery in the control animals. Such observations were not seen in those animals receiving FPL 55618 where, in contrast to controls, numerous intact mast cells were often seen within the tumour mass. Following cessation of the MC-stabilising treatment progressive mast cell activation was evident within 2-4 days, primarily at the tumour periphery. In vitro studies have shown that drug concentrations equivalent to five times the in vivo dose had no effect on the proliferative rate or viability of the MTLn3 cells. Moreover, the proliferative rate of these cells in culture was significantly increased when exposed to soluble mast cell products. Thus our data indicate that a mast cell-stabilising compound has significant benefits in reducing tumour growth in vivo, an observation which supports the concept that mast cell:tumour cell interactions are important for the growth and invasive properties demonstrated by this model of breast carcinoma.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson P. H. Applied biology in developing countries. Science. 1982 Sep 3;217(4563):889–889. doi: 10.1126/science.7112102. [DOI] [PubMed] [Google Scholar]
- Azizkhan R. G., Azizkhan J. C., Zetter B. R., Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980 Oct 1;152(4):931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeza M. L., Reddigari S., Haak-Frendscho M., Kaplan A. P. Purification and further characterization of human mononuclear cell histamine-releasing factor. J Clin Invest. 1989 Apr;83(4):1204–1210. doi: 10.1172/JCI114002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholeyns J., Bouclier M. Involvement of histamine in growth of mouse and rat tumors: antitumoral properties of monofluoromethylhistidine, an enzyme-activated irreversible inhibitor of histidine decarboxylase. Cancer Res. 1984 Feb;44(2):639–645. [PubMed] [Google Scholar]
- Bartholomaeus W. N., Bray A. E., Papadimitriou J. M., Keast D. Immune response to a transplantable malignant melanoma in mice. J Natl Cancer Inst. 1974 Oct;53(4):1065–1072. doi: 10.1093/jnci/53.4.1065. [DOI] [PubMed] [Google Scholar]
- Bartholomaeus W. N., Keast D. Reaginic antibody to tumour and alloantigens in mice. Nat New Biol. 1972 Oct 18;239(94):206–207. doi: 10.1038/newbio239206a0. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Uitto J., Walters R. C., Eisen A. Z. Enhanced collagenase production by fibroblasts derived from human basal cell carcinomas. Cancer Res. 1979 Nov;39(11):4594–4599. [PubMed] [Google Scholar]
- Biswas C. Tumor cell stimulation of collagenase production by fibroblasts. Biochem Biophys Res Commun. 1982 Dec 15;109(3):1026–1034. doi: 10.1016/0006-291x(82)92042-3. [DOI] [PubMed] [Google Scholar]
- Bromley M., Woolley D. E. Histopathology of the rheumatoid lesion. Identification of cell types at sites of cartilage erosion. Arthritis Rheum. 1984 Aug;27(8):857–863. doi: 10.1002/art.1780270804. [DOI] [PubMed] [Google Scholar]
- Broom B. C., Alexander P. Mast cell and anaphylactic antibody responses in inbred rats to syngeneic fibrosarcomas. Int Arch Allergy Appl Immunol. 1975;49(5):627–631. doi: 10.1159/000231444. [DOI] [PubMed] [Google Scholar]
- Broom B. C., Alexander P. Rat tumour allografts evoke anaphylactic antibody responses. Immunology. 1975 Jun;28(6):1033–1040. [PMC free article] [PubMed] [Google Scholar]
- CSABA G., ACS T., HORVATH C., MOLD K. Genesis and function of mast cells. Mast cell and plasmacyte reaction to induced, homologous and heterologous tumours. Br J Cancer. 1961 Jun;15:327–335. doi: 10.1038/bjc.1961.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbous M. K., El-Torky M., Haney L., Brinkley S. B., Sobhy N. Collagenase activity in rabbit carcinoma: cell source and cell interactions. Int J Cancer. 1983 Mar 15;31(3):357–364. doi: 10.1002/ijc.2910310317. [DOI] [PubMed] [Google Scholar]
- Dabbous M. K., Walker R., Haney L., Carter L. M., Nicolson G. L., Woolley D. E. Mast cells and matrix degradation at sites of tumour invasion in rat mammary adenocarcinoma. Br J Cancer. 1986 Sep;54(3):459–465. doi: 10.1038/bjc.1986.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbous M. K., Woolley D. E., Haney L., Carter L. M., Nicolson G. L. Host-mediated effectors of tumor invasion: role of mast cells in matrix degradation. Clin Exp Metastasis. 1986 Apr-Jun;4(2):141–152. doi: 10.1007/BF00119080. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Burd P. R., Galli S. J. Mast cells as a source of multifunctional cytokines. Immunol Today. 1990 Dec;11(12):458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- Graham R. M., Graham J. B. Mast cells and cancer of the cervix. Surg Gynecol Obstet. 1966 Jul;123(1):3–9. [PubMed] [Google Scholar]
- Gruber B. L., Marchese M. J., Suzuki K., Schwartz L. B., Okada Y., Nagase H., Ramamurthy N. S. Synovial procollagenase activation by human mast cell tryptase dependence upon matrix metalloproteinase 3 activation. J Clin Invest. 1989 Nov;84(5):1657–1662. doi: 10.1172/JCI114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit F. Mast cells and metachromasia in human breast cancer: their occurrence, significance and consequence: a preliminary report. J Pathol. 1981 May;134(1):7–11. doi: 10.1002/path.1711340103. [DOI] [PubMed] [Google Scholar]
- Hartveit F., Thoresen S., Tangen M., Maartmann-Moe H. Mast cell changes and tumour dissemination in human breast carcinoma. Invasion Metastasis. 1984;4(3):146–155. [PubMed] [Google Scholar]
- Ionov I. D. Mast cell-basophil system in tumor growth. Neurofibromatosis. 1989;2(4):204–212. [PubMed] [Google Scholar]
- Kessler D. A., Langer R. S., Pless N. A., Folkman J. Mast cells and tumor angiogenesis. Int J Cancer. 1976 Nov 15;18(5):703–709. doi: 10.1002/ijc.2910180520. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. Mediation of local homeostasis and inflammation by leukotrienes and other mast cell-dependent compounds. Nature. 1981 Sep 10;293(5828):103–108. doi: 10.1038/293103a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Thorgeirsson U. P., Garbisa S. Role of collagenases in tumor cell invasion. Cancer Metastasis Rev. 1982;1(4):277–288. doi: 10.1007/BF00124213. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Neri A., Nicolson G. L. Phenotypic drift of metastatic and cell-surface properties of mammary adenocarcinoma cell clones during growth in vitro. Int J Cancer. 1981 Dec;28(6):731–738. doi: 10.1002/ijc.2910280612. [DOI] [PubMed] [Google Scholar]
- Neri A., Welch D., Kawaguchi T., Nicolson G. L. Development and biologic properties of malignant cell sublines and clones of a spontaneously metastasizing rat mammary adenocarcinoma. J Natl Cancer Inst. 1982 Mar;68(3):507–517. [PubMed] [Google Scholar]
- Nicolson G. L. Organ specificity of tumor metastasis: role of preferential adhesion, invasion and growth of malignant cells at specific secondary sites. Cancer Metastasis Rev. 1988 Jun;7(2):143–188. doi: 10.1007/BF00046483. [DOI] [PubMed] [Google Scholar]
- Nordlund J. J., Askenase P. W. The effect of histamine, antihistamines, and a mast cell stabilizer on the growth of cloudman melanoma cells in DBA/2 mice. J Invest Dermatol. 1983 Jul;81(1):28–31. doi: 10.1111/1523-1747.ep12538356. [DOI] [PubMed] [Google Scholar]
- Norrby K., Eneström S. Cellular and extracellular changes following mast-cell secretion in avascular rat mesentery. An electron-microscopic study. Cell Tissue Res. 1984;235(2):339–345. doi: 10.1007/BF00217858. [DOI] [PubMed] [Google Scholar]
- Norrby K. Evidence of mast-cell histamine being mitogenic in intact tissue. Agents Actions. 1985 Apr;16(3-4):287–290. doi: 10.1007/BF01983162. [DOI] [PubMed] [Google Scholar]
- Parwaresch M. R., Horny H. P., Lennert K. Tissue mast cells in health and disease. Pathol Res Pract. 1985 Mar;179(4-5):439–461. doi: 10.1016/s0344-0338(85)80184-9. [DOI] [PubMed] [Google Scholar]
- Roche W. R. Mast cells and tumors. The specific enhancement of tumor proliferation in vitro. Am J Pathol. 1985 Apr;119(1):57–64. [PMC free article] [PubMed] [Google Scholar]
- Roche W. R. The nature and significance of tumour-associated mast cells. J Pathol. 1986 Feb;148(2):175–182. doi: 10.1002/path.1711480208. [DOI] [PubMed] [Google Scholar]
- SCOTT K. G. The mast cell, its amines, and tumor growth in rodents and man. Ann N Y Acad Sci. 1963 Feb 26;103:285–312. doi: 10.1111/j.1749-6632.1963.tb53705.x. [DOI] [PubMed] [Google Scholar]
- SMYTH C. J., GUM O. B. Mast cells in connective tissue diseases. Arthritis Rheum. 1958 Apr;1(2):178–180. doi: 10.1002/art.1780010211. [DOI] [PubMed] [Google Scholar]
- Serafin W. E., Austen K. F. Mediators of immediate hypersensitivity reactions. N Engl J Med. 1987 Jul 2;317(1):30–34. doi: 10.1056/NEJM198707023170106. [DOI] [PubMed] [Google Scholar]
- Starkey J. R., Crowle P. K., Taubenberger S. Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor angiogenesis. Int J Cancer. 1988 Jul 15;42(1):48–52. doi: 10.1002/ijc.2910420110. [DOI] [PubMed] [Google Scholar]
- Suschitzky J. L., Sheard P. The search for antiallergic drugs for the treatment of asthma--problems in finding a successor to sodium cromoglycate. Prog Med Chem. 1984;21:1–61. doi: 10.1016/s0079-6468(08)70406-5. [DOI] [PubMed] [Google Scholar]
- Sweeney W. T., Seibel H. R. Histamine release from peritoneal mast cells of tumor susceptible rats following periods of tumor growth and sensitization with tumor antigens and B. pertussis. Int Arch Allergy Appl Immunol. 1973;45(5):789–794. doi: 10.1159/000231078. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Yoffe J. R., Taylor D. J., Wooley D. E. Mast cell products stimulate collagenase and prostaglandin E production by cultures of adherent rheumatoid synovial cells. Biochem Biophys Res Commun. 1984 Jul 18;122(1):270–276. doi: 10.1016/0006-291x(84)90470-4. [DOI] [PubMed] [Google Scholar]



