Abstract
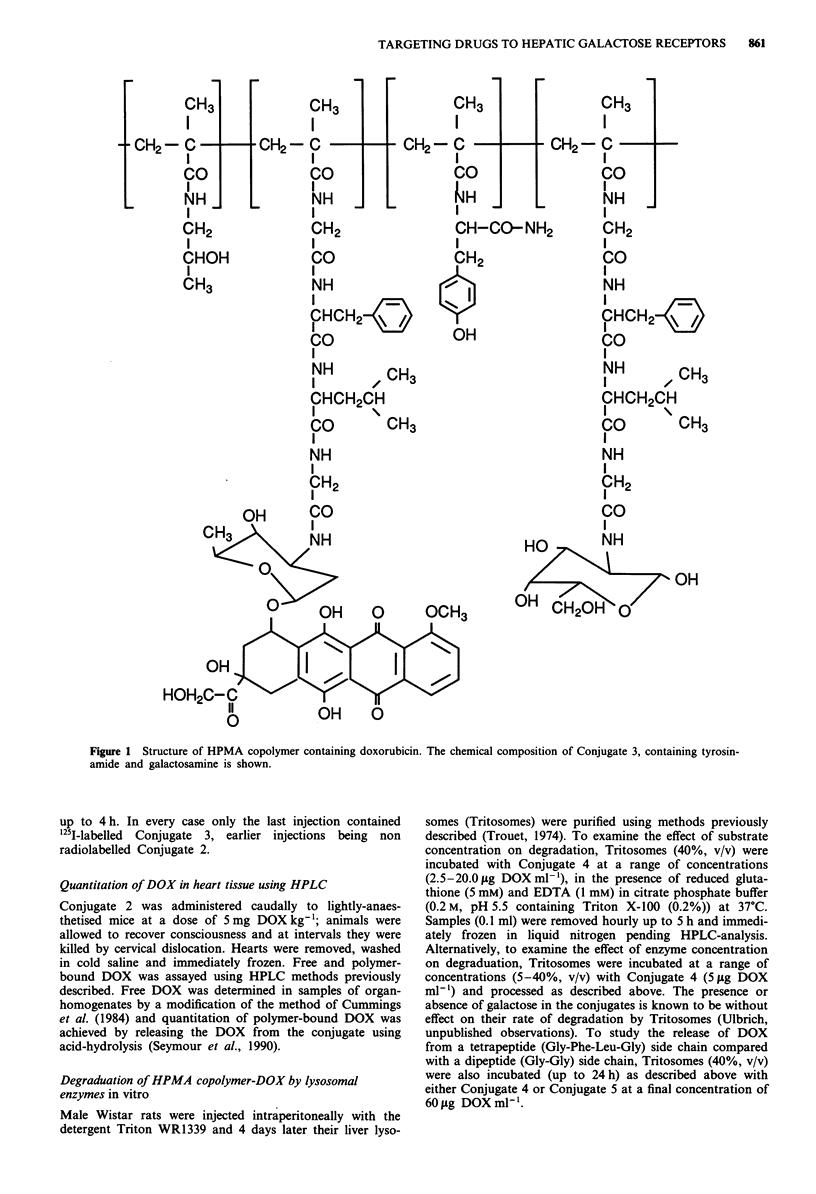
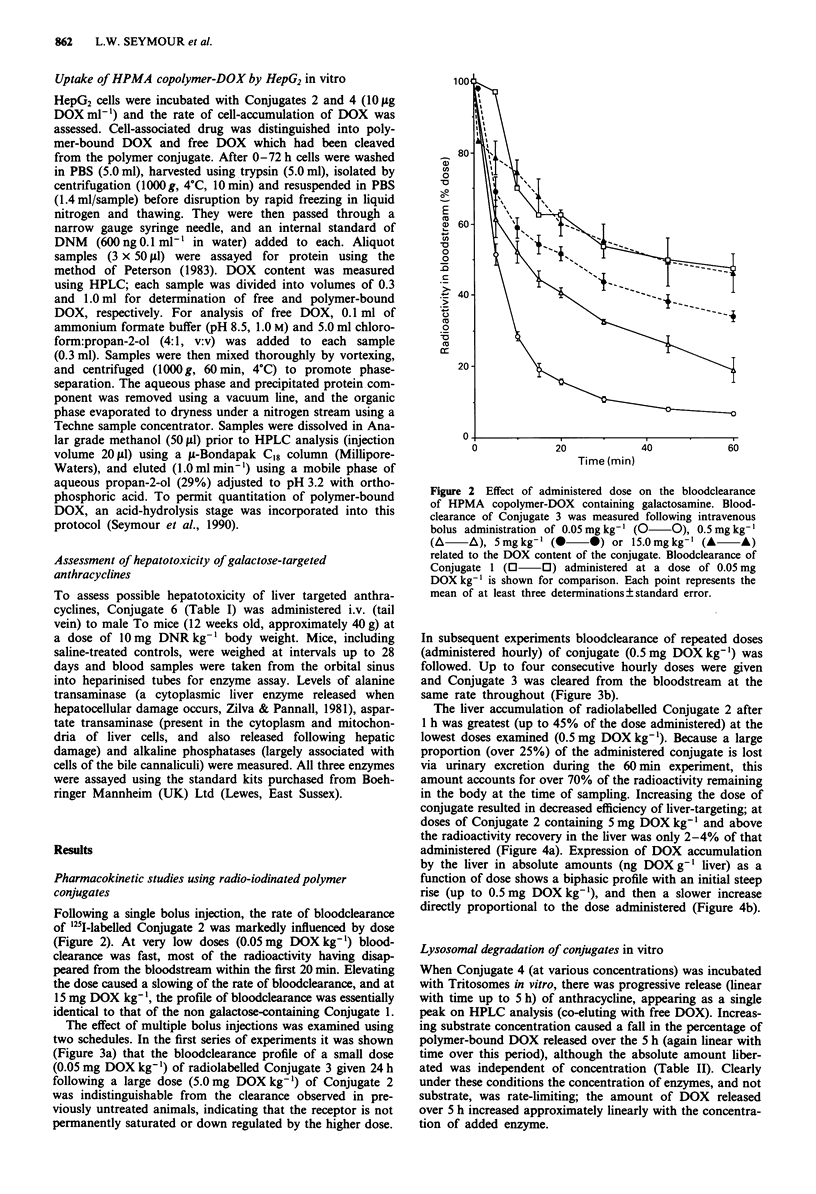
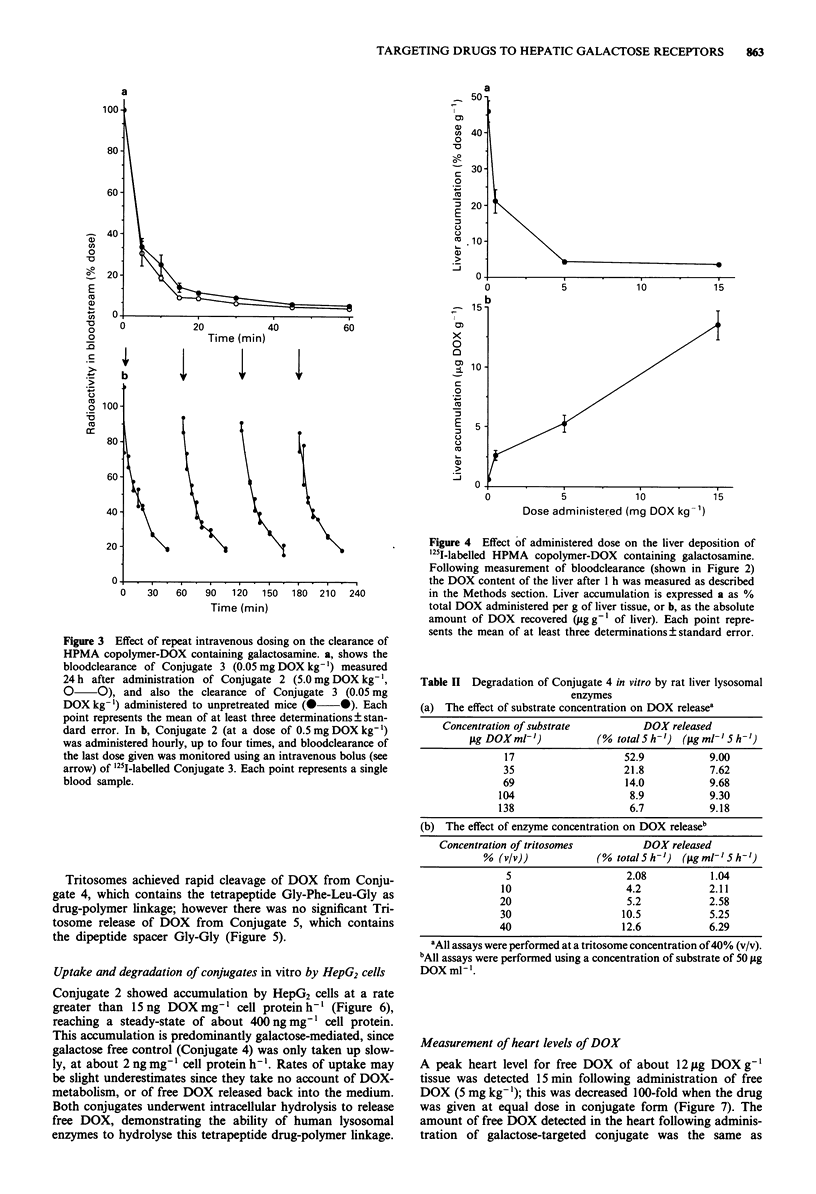
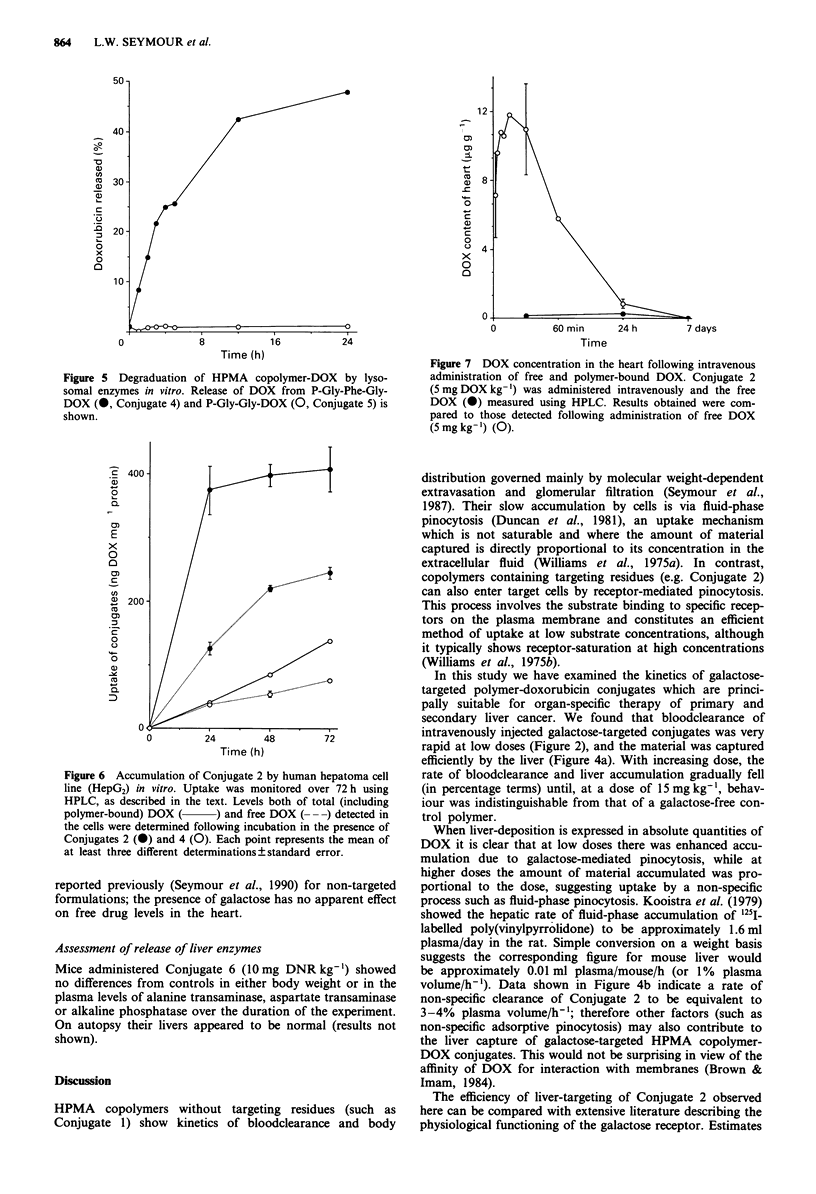
N-(2-Hydroxypropyl)methacrylamide (HPMA) copolymers containing doxorubicin (DOX) and galactosamine can be targeted to the hepatocyte galactose receptor for organ-specific chemotherapy of primary and metastatic liver cancer. Here we report the dose-dependent pharmacokinetics of this macromolecular conjugate. Following intravenous administration to mice most efficient liver targeting was seen at low dose (0.05 mg DOX kg-1), with receptor saturation observed using higher bolus doses. Repeated low dose bolus injections did not cause down-regulation of the galactose receptor and targeted drug delivery rates of greater than or equal to 2 micrograms DOX g-1 liver h-1 were achieved. DOX is released from such conjugates intracellularly via action of lysosomal proteinases. It was shown that isolated rat liver lysosomal enzymes (Tritosomes) can release unmodified DOX from the peptidyl side chain Gly-Phe-Leu-Gly at a rate greater than or equal to 3 micrograms DOX g-1 liver h-1 i.e. the hydrolytic capacity is greater than the observed rate of drug delivery to the liver lysosomes in vivo. Although most conjugate would be captured by normal hepatocytes following intravenous administration, it was shown that the human hepatoma cell line HepG2 retains the galactose receptor, accumulating and processing the conjugate efficiently. Potential dose limiting toxicities of such drug conjugates could include cardio- or hepatotoxicity. Administration of conjugate reduced the 15 min heart level of DOX approximately 100-fold compared with that observed for an equivalent dose of free drug. Preliminary experiments showed that plasma levels of alkaline phosphatase, alanine transaminase and asparate transaminase did not change following administration of HPMA copolymer-daunorubicin (DNR) (10 mg DNR kg-1) indicating no significant heptatoxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cady B. Natural history of primary and secondary tumors of the liver. Semin Oncol. 1983 Jun;10(2):127–134. [PubMed] [Google Scholar]
- Cassidy J., Duncan R., Morrison G. J., Strohalm J., Plocova D., Kopecek J., Kaye S. B. Activity of N-(2-hydroxypropyl)methacrylamide copolymers containing daunomycin against a rat tumour model. Biochem Pharmacol. 1989 Mar 15;38(6):875–879. doi: 10.1016/0006-2952(89)90274-8. [DOI] [PubMed] [Google Scholar]
- Ceulemans F., Baurain R., Geubel A., Lesur B., Rolin-van Swieten D., Trouet A. Pilotage des anthracyclines et hépatomes. Pathol Biol (Paris) 1987 Jan;35(1):61–68. [PubMed] [Google Scholar]
- Chang T. M., Chang C. L. Hepatic uptake of asialoglycoprotein is different among mammalian species due to different receptor distribution. Biochim Biophys Acta. 1988 Jul 7;942(1):57–64. doi: 10.1016/0005-2736(88)90274-x. [DOI] [PubMed] [Google Scholar]
- Cummings J., Stuart J. F., Calman K. C. Determination of adriamycin, adriamycinol and their 7-deoxyaglycones in human serum by high-performance liquid chromatography. J Chromatogr. 1984 Nov 9;311(1):125–133. doi: 10.1016/s0378-4347(00)84698-8. [DOI] [PubMed] [Google Scholar]
- Dragsten P. R., Mitchell D. B., Covert G., Baker T. Drug delivery using vesicles targeted to the hepatic asialoglycoprotein receptor. Biochim Biophys Acta. 1987 Dec 7;926(3):270–279. doi: 10.1016/0304-4165(87)90213-3. [DOI] [PubMed] [Google Scholar]
- Duncan R., Cable H. C., Lloyd J. B., Rejmanová P., Kopecek J. Degradation of side-chains of N-(2-hydroxypropyl)methacrylamide copolymers by lysosomal thiol-proteinases. Biosci Rep. 1982 Dec;2(12):1041–1046. doi: 10.1007/BF01122173. [DOI] [PubMed] [Google Scholar]
- Duncan R., Kopecek J., Rejmanová P., Lloyd J. B. Targeting of N-(2-hydroxypropyl)methacrylamide copolymers to liver by incorporation of galactose residues. Biochim Biophys Acta. 1983 Feb 22;755(3):518–521. doi: 10.1016/0304-4165(83)90258-1. [DOI] [PubMed] [Google Scholar]
- Duncan R., Rejmanova P., Kopecek J., Lloyd J. B. Pinocytic uptake and intracellular degradation of N-(2-hydroxypropyl)methacrylamide copolymers. A potential drug delivery system. Biochim Biophys Acta. 1981 Nov 18;678(1):143–150. doi: 10.1016/0304-4165(81)90058-1. [DOI] [PubMed] [Google Scholar]
- Duncan R., Seymour L. C., Scarlett L., Lloyd J. B., Rejmanová P., Kopecek J. Fate of N-(2-hydroxypropyl)methacrylamide copolymers with pendent galactosamine residues after intravenous administration to rats. Biochim Biophys Acta. 1986 Jan 15;880(1):62–71. doi: 10.1016/0304-4165(86)90120-0. [DOI] [PubMed] [Google Scholar]
- Nerenstone S. R., Ihde D. C., Friedman M. A. Clinical trials in primary hepatocellular carcinoma: current status and future directions. Cancer Treat Rev. 1988 Mar;15(1):1–31. doi: 10.1016/0305-7372(88)90007-2. [DOI] [PubMed] [Google Scholar]
- O'Hare K. B., Hume I. C., Scarlett L., Chytrý V., Kopecková P., Kopecek J., Duncan R. Effect of galactose on interaction of N-(2-hydroxypropyl)methacrylamide copolymers with hepatoma cells in culture: preliminary application to an anticancer agent, daunomycin. Hepatology. 1989 Aug;10(2):207–214. doi: 10.1002/hep.1840100215. [DOI] [PubMed] [Google Scholar]
- Order S. E., Klein J. L., Ettinger D., Alderson P., Siegelman S., Leichner P. Phase I-II study of radiolabeled antibody integrated in the treatment of primary hepatic malignancies. Int J Radiat Oncol Biol Phys. 1980 Jun;6(6):703–710. doi: 10.1016/0360-3016(80)90226-6. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Van Herle A. J., Naruse R. T., Fierer G., Costin A. In vivo quantification of receptor-mediated uptake of asialoglycoproteins by rat liver. J Biol Chem. 1983 Jan 25;258(2):990–994. [PubMed] [Google Scholar]
- Peterson G. L. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- Rejmanová P., Kopecek J., Duncan R., Lloyd J. B. Stability in rat plasma and serum of lysosomally degradable oligopeptide sequences in N-(2-hydroxypropyl) methacrylamide copolymers. Biomaterials. 1985 Jan;6(1):45–48. doi: 10.1016/0142-9612(85)90037-7. [DOI] [PubMed] [Google Scholar]
- Rihova B., Bilej M., Vetvicka V., Ulbrich K., Strohalm J., Kopecek J., Duncan R. Biocompatibility of N-(2-hydroxypropyl) methacrylamide copolymers containing adriamycin. Immunogenicity, and effect on haematopoietic stem cells in bone marrow in vivo and mouse splenocytes and human peripheral blood lymphocytes in vitro. Biomaterials. 1989 Jul;10(5):335–342. doi: 10.1016/0142-9612(89)90075-6. [DOI] [PubMed] [Google Scholar]
- Schlesinger P. H., Rodman J. S., Doebber T. W., Stahl P. D., Lee Y. C., Stowell C. P., Kuhlenschmidt T. B. The role of extra-hepatic tissues in the receptor-mediated plasma clearance of glycoproteins terminated by mannose or N-acetylglucosamine. Biochem J. 1980 Nov 15;192(2):597–606. doi: 10.1042/bj1920597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Fridovich S. E., Lodish H. F. Kinetics of internalization and recycling of the asialoglycoprotein receptor in a hepatoma cell line. J Biol Chem. 1982 Apr 25;257(8):4230–4237. [PubMed] [Google Scholar]
- Seymour L. W., Duncan R., Strohalm J., Kopecek J. Effect of molecular weight (Mw) of N-(2-hydroxypropyl)methacrylamide copolymers on body distribution and rate of excretion after subcutaneous, intraperitoneal, and intravenous administration to rats. J Biomed Mater Res. 1987 Nov;21(11):1341–1358. doi: 10.1002/jbm.820211106. [DOI] [PubMed] [Google Scholar]
- Seymour L. W., Ulbrich K., Strohalm J., Kopecek J., Duncan R. The pharmacokinetics of polymer-bound adriamycin. Biochem Pharmacol. 1990 Mar 15;39(6):1125–1131. doi: 10.1016/0006-2952(90)90293-t. [DOI] [PubMed] [Google Scholar]
- Shapira J., Gotfried M., Lishner M., Ravid M. Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regimen. A prospective randomized evaluation. Cancer. 1990 Feb 15;65(4):870–873. doi: 10.1002/1097-0142(19900215)65:4<870::aid-cncr2820650407>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- The thirtieth annual meeting of the British Association for Cancer Research and the fourth annual meeting of the Association of Cancer Physicians. 10-12 April 1989, Glasgow, UK. Br J Cancer. 1989 Sep;60(3):441–504. [PMC free article] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Virgolini I., Müller C., Klepetko W., Angelberger P., Bergmann H., O'Grady J., Sinzinger H. Decreased hepatic function in patients with hepatoma or liver metastasis monitored by a hepatocyte specific galactosylated radioligand. Br J Cancer. 1990 Jun;61(6):937–941. doi: 10.1038/bjc.1990.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R., Doyle D. Turnover of the surface proteins and the receptor for serum asialoglycoproteins in primary cultures of rat hepatocytes. J Biol Chem. 1981 Feb 10;256(3):1346–1355. [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. II. Kinetics of protein uptake and digestion by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):123–134. doi: 10.1083/jcb.64.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]


