Abstract
We recently reported that fluoxetine or paroxetine, two selective serotonin reuptake inhibitors (SSRIs), when administered to rats, increase the brain content of the neurosteroid 3α-hydroxy-5α-pregnane-20-one (3α5α-ALLO) without altering the brain content of other neurosteroids. ALLO (3α5α and 3α5β isomers) binds with high affinity to various γ-aminobutyric acid (GABA) receptor A subtypes and facilitates the action of GABA at these receptors. We hypothesized that the increase of ALLO brain content induced by treatment with SSRIs could contribute to alleviating the anxiety and dysphoria associated with the symptomatology of major unipolar depression. We measured ALLO content in four cisternal–lumbar fractions of cerebrospinal fluid (CSF) before and 8–10 weeks after treatment with fluoxetine or fluvoxamine in 15 patients with unipolar major depression. The concentration of ALLO (≈40 fmol/ml in each CSF fraction of three control subjects) was about 60% lower in patients with major unipolar depression. However, in the same patients, fluoxetine or fluvoxamine treatment normalized the CSF ALLO content. Moreover, a statistically significant correlation (r = 0.58; P < 0.023; n = 15) existed between symptomatology improvement (Hamilton Rating Scale for Depression scores) and the increase in CSF ALLO after fluoxetine or fluvoxamine treatment. The CSF content of PREG and PROG remained unaltered after treatment and failed to correlate with the SSRI-induced increase of CSF ALLO. The normalization of CSF ALLO content in depressed patients appears to be sufficient to mediate the anxiolytic and antidysphoric actions of fluoxetine or fluvoxamine via its positive allosteric modulation of GABA type A receptors.
Fluoxetine, fluvoxamine, and other selective 5HT reuptake inhibitors (SSRIs) have a spectrum of therapeutic actions that is broader than that of the monoamine oxidase inhibitors or the tricyclic imipramine-like antidepressants (1–5). Because several lines of evidence indicate that the action of various antidepressant classes is related to an enhancement of serotonin (5HT)-mediated neurotransmission and SSRIs are more selective in inhibiting 5HT reuptake than tricyclic antidepressants (6), it is possible that the therapeutic properties that are exclusively elicited by SSRIs may not depend only on 5HT neurotransmission for their action.
We have recently reported that fluoxetine and paroxetine, two SSRIs, but not imipramine, when administered to rats, increase the steady-state brain content of the neurosteroid 3α-hydroxy-5α-pregnane-20-one (3α5α-ALLO), without altering the brain content of other neurosteroids (7) (for chemical structure and biosynthetic pathways of neurosteroids, see Fig. 1).
Figure 1.
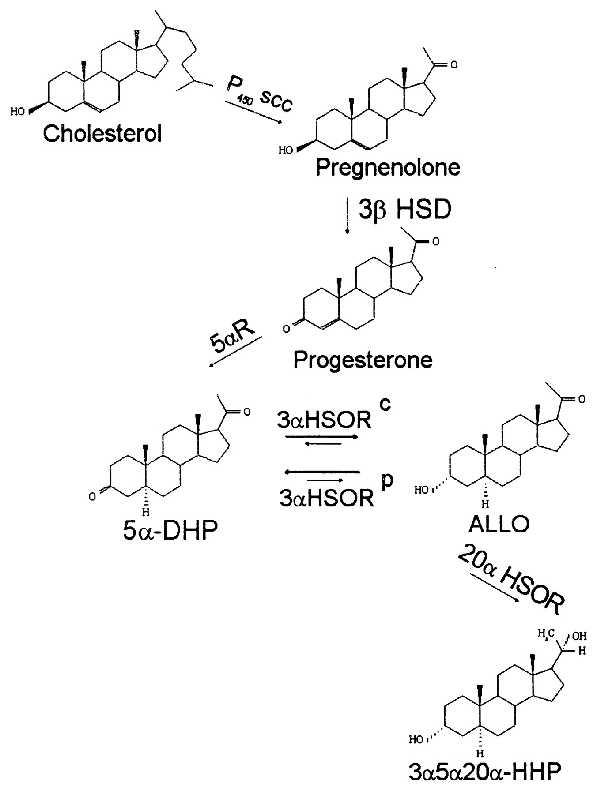
Biosynthesis of neurosteroids. 5α-DHP, 5α-dehydroprogesterone; ALLO, 3α-hydroxy, 5α-pregnane-20-one; 3α5α20α-HHP, 3α,5α,20α-hexahydroprogesterone; P450scc, cytochrome P450 side chain cleavage; 3β-HSD, 3β-hydroxysteroid dehydrogenase; 3α-HSORC, 3α-hydroxysteroid oxidoreductase cytosolic (Km for 5αDHP, 80 nM; Km for ALLO, 2 μM; NADPH/NADP+ linked); 3α-HSORP, 3α-hydroxysteroid oxidoreductase particulate (Km for 5α-DHP, 230 nM; Km for ALLO, 58 nM; NADH/NAD+-linked); 20α-HSOR, 20α-hydroxysteroidoxydoreductase. The kinetic properties of 3α-HSORC and 3α-HSORP are from Karavolas and Hodges (8).
The two stereoisomers, 3α5α-ALLO and 3α-hydroxy-5β pregnane-20-one (3α5β-ALLO), heretofore termed ALLO, in nanomolar concentrations specifically bind to an allosteric center expressed by every γ-aminobutyric acid type A (GABAA) receptor subtype and equipotently facilitate the γ-aminobutyric acid (GABA)-gating of Cl− channels (9–14). Because of their ability to facilitate GABAergic transmission by this mechanism, ALLO belongs to a specific class of steroids termed neurosteroids.
Thus, it is our working hypothesis that if ALLO were to accumulate in the brain of subjects receiving fluoxetine or other SSRIs, it may contribute to alleviating anxiety and dysphoria associated with other symptoms of major unipolar depression or premenstrual dysphoria through a modulatory action at various GABAA receptor subtypes (11–15).
Electrophysiological patch-clamp studies using membrane patches excised from dissociated bovine chromaffin cells demonstrate that ALLO modulates GABAA receptor function only if applied extracellularly (10). This information suggested to us that the increase in ALLO brain content elicited by fluoxetine in rats would be of pharmacological significance only if the ALLO accumulating in the brain of rats receiving fluoxetine could be released from the cells that synthesize ALLO and act on GABAA receptors located on the membrane of adjacent neurons. In experiments designed to address this question (V.U. and A.G., unpublished results), we have found that a relationship exists between fluoxetine efficacy to increase ALLO content in different rat brain areas and the content of this steroid in the corresponding brain microdialysates.
This report shows that the administration of fluoxetine or fluvoxamine for 8–10 weeks to patients affected by major unipolar depression improves the severity of the symptomatology assessed by the Hamilton Rating Scale for Depression (HAM-D) in a manner that correlates with the increase of ALLO cerebrospinal fluid (CSF) content.
METHODS
Subject Selection and Assessment.
Twenty-four subjects (Department of Psychiatry, Washington Univ.) with major unipolar depression provided written informed consent in a study approved by the Washington University Human Subject Committee for the collection of CSF and written informed consent to participate in a clinical trial to assess fluoxetine and fluvoxamine efficacy in the treatment of major depression. Subjects were assessed prior to treatment after a 1- to 2-week observation period with placebo administration and then at weekly intervals during treatment by an experienced clinician using the HAM-D. Inclusion criteria were as follows: age of 18–65 years, Diagnostic and Statistical Manual III Revised (DSM-III-R) diagnosis of major depression, a minimum score of 20 on the 21-item HAM-D, and a minimum score of 2 on the item “depressed mood” at the screening and baseline visits (16). Exclusion criteria were as follows: any comorbid psychiatric illness, substance abuse disorder by DSM-III-R criteria, severe suicide risk, placebo response of 20% or greater improvement on the HAM-D during the observation phase, significant medical illness or laboratory abnormalities, prior hypersensitivity to serotonin reuptake inhibitors, pregnancy or lactation, electroconvulsive treatment within the previous 90 days, treatment with investigational drugs within the previous 30 days, treatment with monoamine oxidase inhibitors or phenothiazines within the previous 14 days, treatment with other psychotropic drugs within the previous 6 days, treatment with fluoxetine within the previous 6 weeks, and previous participation in a fluvoxamine clinical trial.
After the initial 1- to 2-week single blind placebo observation period, all subjects received 8 weeks of double-blind treatment with either fluoxetine or fluvoxamine. The antidepressant dose was titrated to obtain an optimal clinical response with minimum side effects (16). The CSF of three nondepressed patients (two females, 45 and 23 years old, and one male, 51 years old) that provided written informed consent for the collection of CSF were obtained from the Department of Veteran Affairs, Psychiatric Service, New Haven Medical Center, New Haven, CT.
CSF samples were obtained by lumbar puncture for the first time during the last 3 days of the placebo observation period prior to starting antidepressant treatment and again during the last 3 days of SSRI treatment, unless otherwise specified. After overnight bed rest and fasting, lumbar punctures were performed in the lateral decubitus position by an anesthesiologist between the hours of 0800 and 0900. A total of 30 ml of CSF was collected in 13 aliquots (16). For the analysis of neurosteroid concentrations, 0.5-ml aliquots of the 1st, 4th, 6th, and 11th fraction were selected because it was not known whether there would be a gradient in steroid content. After collection, the aliquots were deproteinized with perchloric acid to achieve a final concentration of 0.1 M and stored at −70°C until analysis.
Fifteen of the original 24 subjects gave written informed consent for a second lumbar puncture at the end of the 8- to 10-week study period.
Quantitative Analysis of Neurosteroids.
These measures were performed as described (7) with minor modifications.
Extraction.
Alfaxalone (3–5 fmol) was added as an internal standard either to the CSF samples (100–500 μl) or to a mixture of authentic steroids containing a known amount (1–1,000 fmol) of each steroid to be analyzed, in artificial CSF (145 mM NaCl/2.7 mM KCl/1.0 mM MgCl2/2.0 mM NaH2PO4, pH 7.4). The samples were extracted three times with 4 vol of ethyl acetate. The supernatants were collected, pooled, and lyophilized in preparation for derivatization. The recovery of alfaxalone, ALLO, and other neurosteroids through the extraction procedure was ≈80%.
GC/MS separation and analysis.
After heptafluorobutyric acid anhydride (HFBA) derivatization (50 μl of HFBA in 500 μl of ethyl acetate), the extracts were redissolved in hexane and subjected to GC/MS. The Hewlett–Packard model 5890 gas chromatograph was equipped with a Hewlett–Packard 0.5MS capillary column (30 m; i.d., 0.25 mm; film thickness, 0. 25 μm). Helium was used as a carrier gas.
MS was performed with a Hewlett–Packard 5988-B mass spectrometer operating in the negative ion chemical ionization mode (NICI) with methane as the reaction gas. Samples were injected into the column and equilibrated at a temperature of 50°C at a pressure of 15 psi (1 psi = 6.89 kPa). To concentrate the sample and to reduce the peak width, the pressure was increased to 40 psi. Thereafter, gas pressure was programmed to maintain a flow rate of 1 ml/min. The oven temperature was programmed as follows: (i) increase at a rate of 35°C/min until it reaches 210°C, (ii) steady-state temperature at 210°C for 3 min, and (iii) increase from 210°C to 250°C at a rate of 2.5°C/min. The temperature of 250°C was maintained until the end of the chromatographic run.
In MS, the derivatized steroids of interest when subjected to NICI analysis yielded negative ions in the mass range of m/z 100 to m/z 500.
ALLO stereoisomers, progesterone (PROG), pregnenolone (PREG), allotetrahydrodeoxycorticosterone (THDOC), androsterone, 3α,5α,20α-hexahydroprogesterone (3α5α20α-HHP), and 3α5β20α-HHP were identified in a single GC/MS run (20-min duration) based on their GC retention time, and their structural properties were revealed by their unique mass fragmentation pattern. An example of the resolution power of the GC to separate neurosteroids is given in Fig. 2, where it is shown that the 3α5α- and the 3α5β-ALLO stereoisomers can be easily separated from 3β5α- and 3β5β-ALLO, which elute together. Quantitation was optimized by using mass spectrometry in the selected ion monitoring mode (7), where we focused on the most abundant ion fragment of each steroid derivative, which were m/z 474 and 494 for HFBA-3α5α-, -3α5β-, -3β5α-, and -3β5β-ALLO; 472 and 492 for HFBA-PREG; 490 for HFBA-THDOC; 197 for HFBA-PROG; 446 and 466 for HFBA-androsterone; 213 and 452 for HFBA-3α5α20α-HHP; 213 and 452 for HFBA-3α5β20α-HHP; and 194 and 488 ion fragments for alfaxalone (internal standard).
Figure 2.
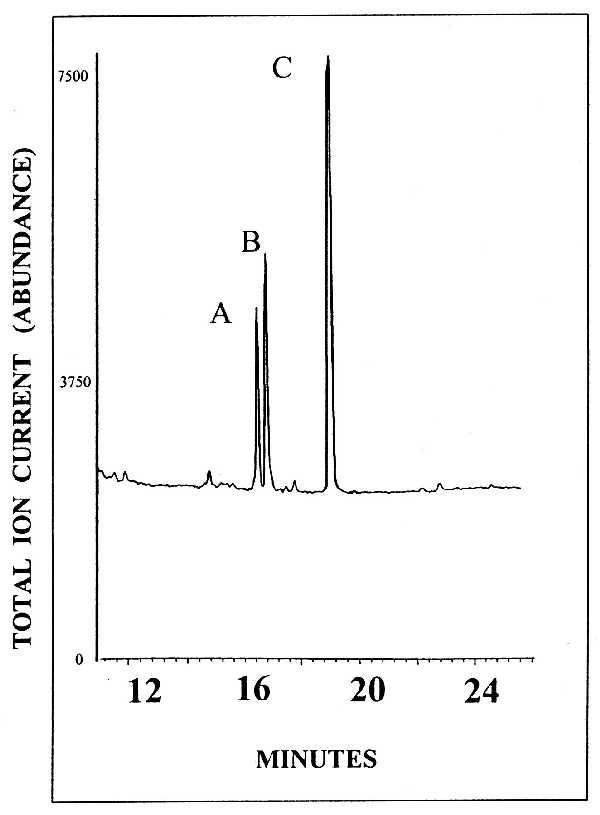
Gas chromographic retention times of ALLO stereoisomers. Peaks: A, HFBA derivative of 3α5α-ALLO; B, HFBA derivatives of 3α5β-ALLO; C, HFBA derivative of 3β5α- and 3β5β-ALLO. The ion current generated by ≈3 pmol of each derivatized steroid is recorded.
The standard curve for the steroid of interest was prepared by combining different known quantities of authentic steroids, from 1 to 1,000 fmol with a constant amount of alfaxalone (3 fmol) as the internal standard. The area under the peak of a known quantity of each steroid was divided by the area under the peak of the internal standard. This ratio was plotted against the quantity of each steroid and used to generate the standard curve. The detection limit for ALLO and for the other steroids studied was ≈10 fmol; the standard curve was linear between 1 and 1,000 fmol.
In establishing the maximal sensitivity of the assay, we considered only peaks with a signal-to-noise ratio greater than 5. The quantity of neurosteroid in the CSF extract was estimated by plotting the ratio of the area under the peak of the neurosteroid to be determined divided by the area under the peak of alfaxalone (internal standard) against similar ratios generated to draw the standard curve. The accuracy of this method was established from the calculated concentrations divided by the actual concentration percentage. The difference between actual and calculated concentrations was less than 2% for each steroid analyzed. Moreover, inter- and intrasample variability was very low (for the reliability and further details of the method, see refs. 7 and 17).
The accuracy of the method was also confirmed by the high correlation existing for PROG (r = 0.90) and PREG (r = 0.91) levels measured in CSF samples obtained before and after fluoxetine or fluvoxamine treatment and by the small (around 10%) standard error of the four-value mean obtained from the analysis in the same patient of the four cisternal–lumbar gradient fractions (Table 1).
Table 1.
Content of ALLO and two of its precursors in CSF of depressed patients before and after treatment with fluoxetine or fluvoxamine
| Patient | Sex | Age, years | Drug
|
PREG, fmol/ml
|
PROG, fmol/ml
|
ALLO, fmol/ml
|
HAM-D | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | mg per kg per day | Before | After | Before | After | Before | After | ||||
| 1 | F | 31 | FX | 0.18 | 94 ± 17 | 110 ± 17 | — | — | 7.0 ± 0.72 | 24 ± 2.2* | 30/1 |
| 2 | F | 29 | FX | 0.68 | 120 ± 21 | 120 ± 14 | 250 ± 11 | 300 ± 10 | 21 ± 3.6 | 32 ± 2.0* | 23/0 |
| 3 | F | 30 | FX | 0.65 | 69 ± 6.9 | 66 ± 7.9 | 570 ± 31 | 350 ± 50 | 13 ± 1.6 | 46 ± 1.4* | 23/1 |
| 4 | M | 46 | FX | 0.34 | — | — | 19 ± 3.8 | 17 ± 4.1 | 12 ± 0.75 | 20 ± 1.0* | 28/4 |
| 5 | F | 41 | FX | 1.5 | 50 ± 3.1 | 41 ± 4.7 | 150 ± 16 | 180 ± 22 | 11 ± 2.1 | 40 ± 1.5* | 25/10 |
| 6 | F | 41 | FX | 0.6 | 60 ± 12 | 53 ± 7.2 | 15 ± 2.8 | 18 ± 3.5 | 30 ± 2.0 | 44 ± 6.8* | 24/14 |
| 7 | M | 37 | FX | 0.27 | 50 ± 4.1 | 41 ± 6.6 | 17 ± 2.5 | 22 ± 5.0 | 12 ± 1.9 | 13 ± 1.9 | 24/23 |
| 8 | M | 51 | FV | 0.54 | 50 ± 4.4 | 70 ± 14 | 29 ± 1.4 | 23 ± 2.1 | 7.2 ± 0.42 | 29 ± 2.5* | 22/3 |
| 9 | F | 23 | FV | 0.95 | 85 ± 25 | 100 ± 4.7 | 44 ± 6.6 | 47 ± 7.2 | 15 ± 1.5 | 72 ± 3.8* | 26/1 |
| 10 | F | 45 | FV | 0.58 | 94 ± 23 | 110 ± 11 | 600 ± 60 | 790 ± 90 | 13 ± 2.6 | 26 ± 4.6* | 23/0 |
| 11 | M | 48 | FV | 0.66 | 85 ± 16 | 66 ± 14 | 66 ± 5.0 | 180 ± 11* | 19 ± 2.8 | 12 ± 0.87 | 30/25† |
| 12 | M | 48 | FV | 0.63 | 38 ± 3.1 | 35 ± 4.4 | 25 ± 6.9 | 19 ± 4.1 | 14 ± 1.6 | 9.2 ± 0.97 | 25/26† |
| 13 | F | 35 | FV | 2.88 | 57 ± 9.8 | 50 ± 13 | 290 ± 31 | 440 ± 27* | 20 ± 1.9 | 54 ± 5.2* | 26/15 |
| 14 | F | 40 | FV | 1.61 | 44 ± 8.5 | 66 ± 25 | 170 ± 31 | 190 ± 41 | 14 ± 1.5 | 30 ± 2.0* | 27/16 |
| 15 | M | 48 | FV | 0.87 | 41 ± 6.9 | 38 ± 5.0 | 23 ± 2.2 | 17 ± 2.5 | 17 ± 1.6 | 19 ± 1.1 | 28/22 |
Steroids were measured in four different cisternal–lumbar CSF fractions in the same patient. Because there was not a significant cisternal–lumbar gradient for ALLO (3α5α-ALLO + 3α5β-ALLO; see Fig. 2), PREG, and PROG, each of the four values obtained by the analysis of the gradient fractions was considered a replication of the same sample. Therefore, for each steroid, we computed the mean ± SEM of four samples for each subject in this table. For each patient, differences before and after treatment were evaluated with an unpaired t test (P < 0.01). FX, fluoxetine; FV, fluvoxamine; F, female; M, male.
Significantly different values before and after treatment.
In these patients, HAM-D scores were obtained seven days before collection of CSF. In all the other patients, the HAM-D scores were obtained a few hours before spinal tap.
Data Analyses.
Neurosteroid levels were determined in four gradient cisternal–lumbar CSF fractions before and after fluoxetine or fluvoxamine treatment. Because levels of ALLO, PROG, and PREG were virtually identical in all four fractions for each patient, these data were pooled and the differences between neurosteroid levels before and after 8–10 weeks of SSRI treatment were evaluated with the use of an unpaired t test (P < 0.05). The relationship between baseline CSF ALLO or other neurosteroid content in each patient and the severity of the depression, expressed by the HAM-D scores before treatment, were quantified with Pearson’s product moment correlation (18). Pretreatment CSF ALLO levels were subtracted from the posttreatment CSF ALLO levels, and the differences were compared with the percent improvement in HAM-D scores also with Pearson’s product moment correlation analyses (18).
RESULTS
By using GC/MF in the NICI mode, we attempted to determine the content of four ALLO stereoisomers (3α5α, 3α5β, 3β5α, and 3β5β), PROG, PREG, THDOC, androsterone, 3α5α20α-HHP, and 3α5β20α-HHP in four CSF cisternal–lumbar gradient fractions obtained from patients with major depression before and after treatment for 8–10 weeks with fluoxetine or fluvoxamine. We could find in these CSF only two of the four ALLO stereoisomers (3α5α and 3α5β), PROG, and PREG but not THDOC, androsterone, 3α5α20α-HHP and 3α5β20α-HHP (detection limit, 1 pM).
Fig. 3 shows that before fluoxetine or fluvoxamine treatment (baseline), the concentrations of the 3α5α- and 3α5β-ALLO stereoisomers were almost identical in all four CSF gradient fractions (fractions analyzed were milliliters 1 and 2, 7 and 8, 11 and 12, and 25 and 26).
Figure 3.
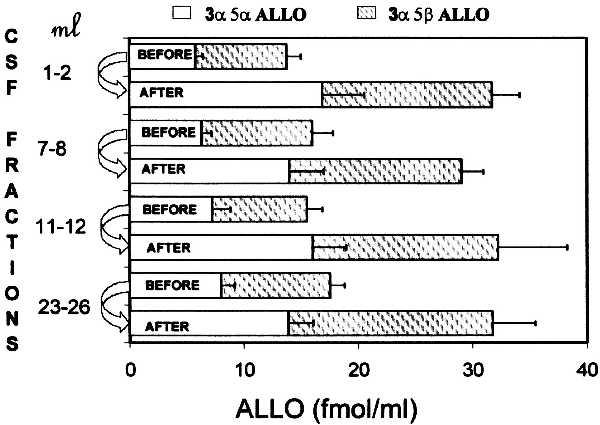
ALLO levels in four cisternal–lumbar gradient fractions of CSF before and 8–10 weeks after fluoxetine or fluvoxamine treatment. Each value is the mean ± SEM of data from 15 depressed patients (see Table 1 for details). ALLO (fmol/ml) includes the level of 3α5α-ALLO (solid bars) and of 3α5β-ALLO (shaded bars).
The stereoisomers 3α5α-ALLO and 3α5β-ALLO have similar potency and efficacy as positive modulators of GABA action at GABAA receptors (9, 10), their CSF concentrations at baseline are similar (Fig. 3), and the extent of the increase elicited by fluoxetine or fluvoxamine treatment is also similar (Fig. 3). Hence we have used the combined concentrations of these neuroactive steroids under the acronym ALLO for statistical analyses of significance and to establish correlations.
Fig. 4 shows that the CSF content of ALLO in three normal subjects (mean ± SEM, 39 ± 4.9 fmol/ml) was higher than the average ALLO content observed in the CSF of depressed patients (15 ± 1.5 fmol/ml; t = 6.0; df = 16; P < 0.0001) before treatment. For the total sample, a statistically significant negative correlation (r = −0.82; P = 0.001) was found between the severity of the depression and CSF ALLO levels (Fig. 4). However, when the group of depressed subjects was considered separately, a significant correlation was not observed.
Figure 4.
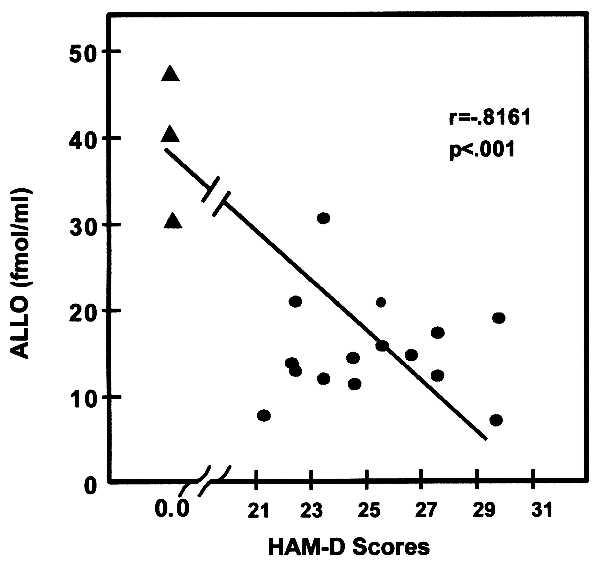
Negative correlation exists between the severity of depression (HAM-D) and ALLO levels in CSF at base line [r = 0.081; P = 0.01; Pearson’s Product Moment Correlation (18)]. •, ALLO levels in CSF of depressed patients; ▴, ALLO level in CSF of control subjects. Each point is the mean of determinations of ALLO in four CSF fractions (see Fig. 2).
Fluoxetine (0.27–1.5 mg per kg per day for 8–10 weeks) doubled ALLO content in all CSF gradient fractions, an average increase of 16 ± 4.2 fmol/ml (±SEM) from 15 ± 2.9 fmol/ml to a final average of 31 ± 4.7 fmol/ml. This increase is highly significant (t = 3.9; df = 6; P = 0.008) and shows a dose-related trend (r = 0.67; P = 0.05; one-tailed test).
Fluvoxamine (0.54–2.88 mg per kg per day for 8–10 weeks) also significantly increased CSF ALLO level by 16 ± 7.0 fmol/ml from a mean baseline value of 15 ± 1.4 fmol/ml to a mean final value of 31 ± 7.5 fmol/ml (t = 2.2; df = 7; P = 0.07). However, the drug dose did not correlate significantly with the increase in ALLO levels (r = 0.38; P = 0.17; one-tailed test).
At baseline, females had a slightly, but not significantly, higher ALLO level than males: 16 ± 2.3 fmol/ml vs. 13 ± 1.7 fmol/ml, respectively (see Table 1), but females received higher doses per kg of SSRIs than males (see Table 1), and after SSRI treatment, the females’ CSF ALLO levels were almost three times higher than those of males: 41 ± 5.0 fmol/ml vs. 17 ± 3.0 fmol/ml (t = 3.6; df = 13; P = 0.003). Moreover, females were younger than males (35 vs. 46 years; t = 3.4; P = 0.005). Analysis of covariance adjusting for age, the drug used, and drug dosage failed to show a significant sex effect (F = 1.9; df = 1,9; P = 0.20).
At baseline, patients had moderate to severe depression (average HAM-D scores = 26 ± 0.32). After SSRI treatment, most patients improved (see Table 1). The average HAM-D score at the end of the study was 11 ± 1.3. The symptoms of six of seven fluoxetine-treated patients were ameliorated by the drug, five remitted fully, and one patient experienced some improvement. It is important to mention that the nonresponder (patient 7) received a very low dose of fluoxetine, which might have been inadequate (Table 1). Three of the eight fluvoxamine-treated patients had a full response, two were partial responders, and three (patients 11, 12, and 15) were nonresponders. This type of response rate is typical for antidepressants. Fig. 5 shows that in patients who responded, the increase in ALLO CSF level elicited by fluoxetine or fluvoxamine could be correlated in a statistically significant manner with the percent of improvement in the HAM-D scores (r = 0.58; df = 15; P = 0.023).
Figure 5.
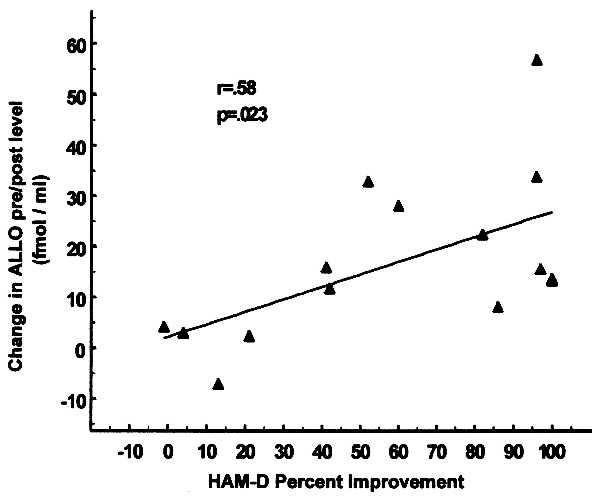
Fluoxetine- or fluvoxamine-elicited increase in the ALLO content of CSF correlates with the improvement in the HAM-D score [r = 0.58; P < 0.023; Pearson’s Product Moment Correlation (18)]. The percent changes in HAM-D scores before and after fluoxetine and fluvoxamine treatment (abscissa) were plotted against changes in corresponding ALLO levels in the CSF (ordinate). For each patient the change in ALLO levels in the CSF before and after treatment reflects the mean of the difference between ALLO level before and after SSRI treatment in the four cisternal–lumbar CSF fractions.
PROG and PREG were measured in the same CSF fractions in which ALLO was determined and were found to be present in the picomolar concentration range and to be similar in every gradient fraction studied (data not shown). The PREG concentrations failed to increase after fluoxetine and fluvoxamine treatment (Table 1). The concentration of PROG also failed to increase after SSRIs treatment (see Table 1) with the exception of subject 11, a 48-year-old male, and subject 13, a 35-year-old female, who had an increase after fluvoxamine treatment (Table 1). CSF PROG levels were higher in female than in male patients. PROG in females before treatment was 260 ± 77 fmol/ml and became 290 ± 87 fmol/ml after SSRI treatment. PROG in males was 30 ± 7 fmol/ml before SSRIs treatment and 46 ± 27 fmol/ml after treatment. The difference between males and females is statistically significant (t = 3.0 and P = 0.002 before treatment; t = 2.3 and P = 0.04 after treatment; df = 12). However, PROG concentrations in CSF of females were not always greater than those of males. Subjects 6 and 9 (females of 41 and 23 years old) had PROG levels similar to those of males. We cannot establish from our study whether the CSF levels of PROG in females correlate with the menstrual cycle because when these CSF samples were collected the ovulatory cycle phases were not recorded. It must be noted, however, that the level of PROG in CSF appears to be unrelated to that of ALLO (Table 1, subjects 4, 6, 8, and 9). Moreover, in subject 3, the PROG level decreases after fluoxetine treatment whereas that of ALLO increases.
We could not find a significant correlation between levels of PROG and PREG before and after treatment (before treatment, r = 0.44, df = 13, and P = 0.13; after treatment, r = 0.50, df = 13, and P = 0.08). Both at baseline and after drug treatment, the CSF content of 3α5α20α-HHP and 3α5β20α-HHP, which are the metabolites of 3α5α- and 3α5β-ALLO, respectively (see Fig. 1), were below the method’s detection limits (≈1 pM). THDOC and androsterone also were never detected.
DISCUSSION
The most significant finding of this study is that in 15 unipolar depressed patients from whom CSF samples were obtained before and 8–10 weeks after fluoxetine or fluvoxamine treatment, the content of both 3α5α- and 3α5β-ALLO stereoisomers was significantly increased. Importantly, we also observed that the largest increase in the CSF content of ALLO occurred in patients who had the greatest improvement in HAM-D scores, whereas patients who failed to improve after SSRI treatment also failed to show a significant increase in CSF content of ALLO. Interestingly, in patients treated with SSRIs, the CSF content of other neurosteroids (i.e., PROG and PREG) failed to change.
Although at the present time we have studied only three control subjects (without mood disorders), the level of ALLO in the CSF of patients with major depression was lower than that of our control subjects. As mentioned above, ALLO is a potent positive modulator of GABA-gated Cl− current intensity (12, 13). Because GABAergic tone may be involved in the pathophysiology of anxiety, it follows that if CSF ALLO is decreased in depression and is normalized after SSRI treatment (Table 1), then this change might have a role in decreasing the anxiety expressed in mood disorders. It must be noted, however, that the concentration of ALLO in CSF of patients treated with SSRIs is relatively low (around 30 pM) and the concentration of ALLO needed to modulate GABAA receptors is in the low nanomolar range (12, 13). We do not have available data on the brain neurosteroid content of our patients, but from rat microdialysate studies, we have estimated that in the spinal fluid the concentration of ALLO accounts for about 10% of the brain ALLO concentration (2.2–3.5 nM) (19). If we extrapolate the rat data (19) to the human brain, we can expect that the average concentration of ALLO in human brain ranges from 3 to 5 nM. Because not all the central nervous system cells synthesize ALLO (15), one can infer that ALLO concentrations will be higher in cells that secrete ALLO around GABAergic synapses, and these concentrations may be sufficient to positively modulate GABAA receptor function.
In the past, major analytical limitations to measuring ALLO with the required sensitivity and specificity have precluded a study of the putative role that this neurosteroid may have in the regulation of mood disorders.
The ALLO isoforms that contain a hydroxyl group in the 3α position (3α5α and 3α5β) are potent positive modulators of GABA action at GABAA receptors, whereas the 3β-ALLO isoforms (3β5α and 3β5β) are inactive (9, 10).
Herein, by using GC/MS in the NICI mode (7), we have been able to isolate and measure femtomole quantities of all four ALLO isoforms, with an inherent identification of the chemical structure, and also, we have demonstrated that the CSF of normal subjects contains picomolar concentrations of the positive allosteric modulators of GABAA receptors 3α5α- and 3α5β- but not 3β5α- or 3β5β-ALLO, both of which are below the detection limit (1 pM).
Evidence for the presence in the mammalian brain of 5α-reductase and 3α-hydroxysteroid oxidoreductase (3α-HSOR), the enzymes that synthesize 5α-dihydroprogesterone (5α-DHP) from PROG and 3α5α-ALLO from 5α-DHP, respectively, is available (Fig. 1 and ref. 8). But the presence of 5β-reductase and 3β-HSOR in the human brain has never been investigated. In the rat brain, the predominant ALLO isoform is 3α5α (8). So far, 3β5β reduced PROG metabolites have been demonstrated only in the brains of birds and human plasma (8, 20).
As we have mentioned earlier (see Table 1), the increase in the content of 3α5α- and 3α5β-ALLO in the CSF after fluoxetine or fluvoxamine treatment is not paralleled by changes in PREG or even PROG. These two steroids can reach the brain after being synthesized in peripheral organs and may accumulate intracellularly in brain.
Interestingly, PROG levels in the CSF vary considerably from individual to individual, and although they are significantly higher in females than in males, individual or sex differences in PROG levels are not reflected in the differences in the CSF levels of ALLO, which at baseline are similar in both depressed males and females (Table 1). This suggests that the brain content of PROG is not a rate-limiting factor for brain ALLO biosynthesis in humans.
Because we do not have pertinent information on the ovulatory cycle of the females patients when the CSF was taken, we must address the question of whether ALLO levels are correlated with changes of circulating PROG levels during the menstrual cycle. To the best of our knowledge, with the exception of the data reported in our study, there are no other studies on the correlation of PROG and ALLO in CSF. However, there are other studies on the correlation of blood levels of ALLO and PROG: Smith et al. (20) and Wang et al. (21) demonstrated that ALLO plasma levels were correlated with PROG plasma levels. Peripherally administered 5α-DHP, the immediate precursor of ALLO (see Fig. 1), and PROG accumulate in the brain; both steroids can be converted into ALLO in the brain (17, 22). However, the rates of the conversion of PROG to 5α-DHP and 5α-DHP to ALLO in the brain depend on the kinetic properties of the pertinent enzymes: PROG is converted to 5α-DHP by the action of type 1 (the most abundant isoform in brain) or type 2 5α-reductase (Fig. 1). Both enzyme isoforms depend on NADPH and exhibit a Km for PROG of 40 and 500 nM, respectively (23). The plasma concentration of PROG oscillates from about 3 to 30 pmol/ml of blood at various time of the cycle (24). Experiments in rats suggest that the brain content of PROG should not exceed significantly that of blood (8). Because the rate of conversion of PROG into 5α-DHP depends on the brain concentration of PROG and the brain contains mostly type 1 5α-reductase with an affinity of 0.5 μM for PROG (23), it is difficult for the brain PROG to reach enzyme saturation during the various phases of the menstrual cycle. The lack of interdependence between PROG and ALLO concentrations observed in the CSF samples of our group of patients with major depression (see Table1) may also be explained by considering the characteristics of 3α HSOR isoforms, the enzymes that catalyze the conversion of 5α-DHP to ALLO and vice versa the conversion of ALLO to 5α-DHP (Fig. 1). Both isoforms, the 3α-HSOR particulate (3α-HSORP) NADH/NAD+-linked (Km for 5α-DHP, 230 nM; Km for ALLO, 58 nM) and the 3α-HSOR cytosolic (3α-HSORC) NADPH/NADP+-linked (Km for 5α-DHP, 80 nM; Km for ALLO, 2 μM) enzymes are present in brain (8). Hence, one can easily understand that the PROG and ALLO concentration can be independent and that the concentration of ALLO can be increased by a selective inhibition of the NADH-linked cell-membrane-bound 3α-HSORP by fluoxetine and fluvoxamine competing with ALLO substrate. Because we have only shown that in vitro fluoxetine increases ALLO concentrations when added to brain slices (7), this putative mechanism of fluoxetine action on ALLO CSF levels requires further investigation.
Several lines of evidence indicate that, in depressed patients, the levels of circulating glucocorticosteroids and presumably PROG are increased and they normalize with treatment (25). Thus, one would expect that if brain ALLO is originating from PROG of peripheral origin, the ALLO content of the CSF would be elevated in depressed patients at baseline and decreased after fluoxetine or fluvoxamine treatment. Our results, however indicate the opposite. Hence, these data further support the notion that ALLO in the CSF might be controlled independently of the pituitary mechanisms controlling PROG formation in the adrenal cortex or other peripheral steroidogenic tissues.
Whether the mechanism(s) whereby fluoxetine or fluvoxamine increase the human CSF level of ALLO involve 5HT remains to be elucidated. Indirect evidence that 5HT reuptake inhibition might not be involved in the SSRI-induced increase of CSF ALLO is as follows: (i) in rats, fluoxetine or paroxetine but not imipramine in doses that completely inhibit brain 5HT reuptake in vitro increases the content of ALLO in brain (7); (ii) in rats and mice treated with para-chloro-phenylalanine to deplete 5HT stores, the fluoxetine-induced increase in the content of ALLO in brain persists unabated (V.U. and A.G., unpublished results). Thus, these findings suggest that 5HT synthesis and storage in nerve terminals is not required for the increase of ALLO elicited by fluoxetine and other SSRIs.
We have established (7) that the mechanism by which fluoxetine increases the content of ALLO in brain does not involve the activation of (i) the P450scc enzyme, which favors the conversion of cholesterol to PREG (Fig. 1) and is under the control of the mitochondrial benzodiazepine receptors; (ii) 3β-hydroxysteroid dehydrogenase, the enzyme that transforms PREG into PROG (Fig. 1); or (iii) the activation of 5α-reductase, the enzyme that transforms PROG into 5α-DHP (Fig. 1). It is notable, however, that after fluoxetine treatment, the content of 5α-DHP, the precursor of ALLO (Fig. 1), is decreased whereas that of ALLO is increased in most brain regions of adrenalectomized castrated rats (7). In vitro experiments, in which the effect of fluoxetine on ALLO biosynthesis or degradation was studied in C6 glioma cells (a cell-line presumably incapable of synthesizing 5HT), suggest that fluoxetine alters ALLO levels by reducing its metabolism rate (7). This suggests that the mechanisms by which fluoxetine and other SSRIs increase the content of ALLO in brain might be at the level of the particulate- NAD+-linked 3α-HSOR isoenzyme using ALLO as the substrate and inhibiting the conversion of ALLO to 5α-DHP.
Acknowledgments
We thank Dr. J. H. Mendelson (Massachusetts General Hospital, Harvard Medical School), Dr. S. M. Paul (Lilly Research Laboratory, Indianapolis), and Dr. R. M. Post (Biological Psychiatry Branch, National Institute of Mental Health) for their constructive criticisms and suggestions. This work is supported in part from Research Grants MH49486-05 and MH56890-01 (to A.G.) MH01370 and 5M01RR0036 (to Y.S.).
ABBREVIATIONS
- SSRI
selective serotonin reuptake inhibitor
- ALLO
3α-hydroxy-5α(β)pregnane-20-one
- GABA
γ-aminobutyric acid
- GABAA
GABA type A receptor
- CSF
cerebrospinal fluid
- PREG
pregnenolone
- PROG
progesterone
- 5HT
serotonin
- HAM-D
Hamilton Rating Scale for Depression
- HFBA
heptafluorobutyric acid anhydride
- NICI
negative ion chemical ionization
- THDOC
allotetrahydrodeoxycorticosterone
- HHP
hexahydroprogesterone
- HSOR
hydroxysteroid oxidoreductase
- DHP
dihydroprogesterone
References
- 1.Eriksson E, Heldberg M A, Andersch B, Sundblad C. Neuropsychopharmacology. 1995;12:167–176. doi: 10.1016/0893-133X(94)00076-C. [DOI] [PubMed] [Google Scholar]
- 2.Janicak P G, Davis J M, Preskorn S H, Ayd F J., Jr . In: Principles and Practice of Psychopharmacotherapy. Gay S, editor. Baltimore: Williams & Wilkins; 1997. pp. 243–294. [Google Scholar]
- 3.Steiner M, Steinberg S, Stewart D, Carter D, Berger C, Reid R, Grover D, Streiner D. N Engl J Med. 1995;332:1529–1534. doi: 10.1056/NEJM199506083322301. [DOI] [PubMed] [Google Scholar]
- 4.Su T P, Schmidt P J, Danaceau M A, Tobin M B, Rosenstein D L, Murphy D L, Rubinow D R. Neuropsychopharmacology. 1997;16:346–356. doi: 10.1016/S0893-133X(96)00245-X. [DOI] [PubMed] [Google Scholar]
- 5.Yonkers K A, Halbreich V, Freeman E, Brown C, Pearlstein T. Psychopharmacol Bull. 1996;32:41–46. [PubMed] [Google Scholar]
- 6.Blier P, deMontigny C. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 7.Uzunov D P, Cooper T B, Costa E, Guidotti A. Proc Natl Acad Sci USA. 1996;93:12599–12604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karavolas H J, Hodges D R. In: Neurosteroids and Brain Functions. Costa E, Paul S M, editors. New York: Thieme; 1991. pp. 135–145. [Google Scholar]
- 9.Gee K W, McCauley L D, Lan N C. Crit Rev Neurobiol. 1995;9:207–227. [PubMed] [Google Scholar]
- 10.Lambert J J, Belelli D, Hill-Venning C, Peters J. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 11.Paul S M, Purdy R H. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- 12.Puia G, Santi M R, Vicini S, Pritchett D B, Purdy R H, Paul S M, Seeburg P H, Costa E. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- 13.Puia G, Ducic I, Vicini S, Costa E. Receptors Channels. 1993;1:135–142. [PubMed] [Google Scholar]
- 14.Zhu W J, Vicini S. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costa E, Cheney D L, Grayson D R, Korneyev A, Longone P, Pani L, Romeo E, Zivkovic E, Guidotti A. Ann NY Acad Sci. 1994;746:223–242. doi: 10.1111/j.1749-6632.1994.tb39240.x. [DOI] [PubMed] [Google Scholar]
- 16.Sheline Y, Bargett M E, Csernansky J G. J Clin Psychopharmacol. 1997;17:11–14. doi: 10.1097/00004714-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Cheney D L, Uzunov D P, Costa E, Guidotti A. J Neurosci. 1995;15:4641–4650. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sokal R R, Rohlf F J. In: Biometry. Wilson J, editor. New York: Freeman; 1981. pp. 565–572. [Google Scholar]
- 19.Guidotti A, Uzunov D P, Auta J, Costa E. In: The Brain: Source and Target for Sex Steroid Hormones. Genazzani A R, Petraglia F, Purdy R H, editors. NY: Parthenon; 1996. pp. 25–41. [Google Scholar]
- 20.Schmidt P J, Purdy R H, Moore P H, Jr, Paul S M, Rubinow D R. J Clin Endocrinol Metab. 1994;79:1256–1260. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- 21.Wang M, Seippel L, Purdy R H, Backstrom T. J Clin Endocrinol Metab. 1996;81:1076–1082. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- 22.Robel P, Young J, Corpechot C, Mayo W, Perche F, Haug M, Simon H, Baulieu E E. J Steroid Biochem Mol Biol. 1995;53:355–360. doi: 10.1016/0960-0760(95)00074-a. [DOI] [PubMed] [Google Scholar]
- 23.Normington K, Russell D W. J Biol Chem. 1992;267:19548–19554. [PubMed] [Google Scholar]
- 24.Rapkin A J, Morgan M, Goldman L, Darrel R N P, Brann W, Simone D, Mahesh V B. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- 25.Barden N, Reul J M, Holsboer F. Trends Neurosci. 1995;18:6–11. doi: 10.1016/0166-2236(95)93942-q. [DOI] [PubMed] [Google Scholar]


