Abstract
The binding protein (BiP; a member of the heat-shock 70 family) is a major chaperone of the endoplasmic reticulum (ER). Interactions with BiP are believed to inhibit unproductive aggregation of newly synthesized secretory proteins during folding and assembly. In vitro, BiP has a preference for peptide sequences enriched in hydrophobic amino acids, which are expected to be exposed only in folding and assembly intermediates or in defective proteins. However, direct information regarding sequences recognized in vivo by BiP on real proteins is very limited. We have shown previously that newly synthesized monomers of the homotrimeric storage protein phaseolin associate with BiP and that phaseolin trimerization in the ER abolishes such interactions. Using different phaseolin constructs and green fluorescent protein (GFP) fusion proteins, we show here that one of the two α-helical regions of polypeptide contact in phaseolin trimers (35 amino acids located close to the C terminus and containing three potential BiP binding sites) effectively promotes BiP association with phaseolin and with secretory GFP fusions expressed in transgenic tobacco or in transfected protoplasts. We also show that overexpressed BiP transiently sequesters phaseolin polypeptides. We conclude that one of the regions of monomer contact is a BiP binding determinant and suggest that during the synthesis of phaseolin, the association with BiP and trimer formation are competing events. Finally, we show that the other, internal region of contact between monomers is necessary for phaseolin assembly in vivo and contains one potential BiP binding site.
INTRODUCTION
Secretory proteins are inserted cotranslationally into the endoplasmic reticulum (ER), the gateway of the secretory pathway. In the ER, newly synthesized polypeptides undergo folding and multimeric proteins assemble (for review, see Vitale and Denecke, 1999). Once these structural maturations have been completed, secretory proteins are transported to the cell surface or the vacuoles (lysosomes in animal cells), in most cases via the Golgi complex. Several helper proteins that reside in the ER assist folding and assembly. The binding protein (BiP) is one such resident and a member of the heat-shock 70 family of molecular chaperones (for review, see Gething, 1999). BiP transiently binds many (perhaps all) newly synthesized polypeptides in the ER lumen and is believed to increase the efficiency of the folding and assembly of secretory proteins by inhibiting unproductive and irreversible aggregation during these processes. BiP contains an N-terminal ATPase domain and a C-terminal peptide binding domain (Gething, 1999). ATP binding to the N-terminal domain triggers the release of the bound peptide from the binding site. BiP also is implicated in protein translocation and in sealing the lumenal side of the ER translocon when this is not active and during the integration of transmembrane proteins into the ER membrane (Hamman et al., 1998; Haigh and Johnson, 2002). Finally, BiP interacts more extensively with structurally defective polypeptides and plays a role in their retrotranslocation into the cytosol for disposal. BiP can avoid the aggregation of such defective proteins, probably facilitating their retrotranslocation through the rather narrow translocation pore (Nishikawa et al., 2001), although it also can become part of stable aggregates when massive synthesis of misfolded proteins occurs (Sparvoli et al., 2000).
One of the features of BiP is its ability to recognize polypeptides only when these are unfolded, misfolded, or, in the case of multimeric proteins, unassembled (Gething, 1999; Vitale and Denecke, 1999). Thus, the peptide binding domain is able to bind a wide variety of nascent polypeptides without recognizing a specific amino acid sequence; at the same time, it can discriminate between native and unfolded structures. Structural studies of the peptide binding site of the bacterial BiP homolog DnaK have revealed that the chaperone can bind polypeptide chains in an extended conformation (Zhu et al., 1996).
In vitro experiments using synthetic random sequences showed that BiP preferentially recognizes peptides with a high content of hydrophobic residues (Flynn et al., 1991). The minimal length recognized was a heptapeptide (Flynn et al., 1991), with the hydrophobic residues present at least at four alternate positions (Blond-Elguindi et al., 1993). The hydrophobic nature of the recognized sequences agrees with the proposed role of BiP in vivo, because such sequences probably are exposed on the protein surface only in folding and assembly intermediates.
The BiP Score program was developed to identify potential BiP binding sites. This program was based initially on results from the in vitro affinity panning of a peptide phage display library (Blond-Elguindi et al., 1993) and was refined using data from the in vitro interaction of BiP with peptides synthesized based on the sequences of immunoglobulin chains and gp160 of Human immunodeficiency virus (Knarr et al., 1995, 1999). The main parameter for the evaluation of affinity for BiP was the ability of a given peptide to stimulate in vitro the ATPase activity of the chaperone. In immunoglobulin heavy and light chains (Knarr et al., 1995; Augustine et al., 2001), in gp160 (Knarr et al., 1999), and in the lysosomal hydrolase N-acetylglucosamine-4-sulfatase (Bradford et al., 1999), most of the predicted potential sites of BiP binding involve interdomain or subunit contact sites or hydrophobic residues that are buried within the folded protein, as revealed by crystallography. However, at present, there is scarce evidence regarding whether these predictions apply to in vivo situations: direct information on sequences recognized in vivo on real proteins, in any eukaryotic system, is very limited.
The expression of truncated immunoglobulin polypeptides shows that the C1 constant domain of heavy chains and the variable domain of light chains determine the in vivo BiP binding to unassembled subunits (Hendershot et al., 1987; Skowronek et al., 1998). Each of these domains is ∼110 amino acid long. Another region of BiP binding in vivo has been identified in one of the surface proteins of Hepatitis B virus. This has been demonstrated by showing that BiP interacts in vivo with the large (L) but not with the middle (M) surface protein (Cho et al., 2003). The two proteins are synthesized by the host's secretory pathway and are highly related: they differ only because the L protein has an N-terminal extension (pre-S1 sequence; 108 or 119 residues, depending on the subtype) absent from the M protein. Therefore, the pre-S1 sequence is a BiP binding determinant. This segment also is recognized by BiP in vitro (Cho et al., 2003).
In the present work, we have attempted to identify in vivo the BiP binding regions in a plant protein, phaseolin. Phaseolin is a homotrimeric vacuolar storage glycoprotein. Phaseolin trimers are held together mainly by hydrophobic interactions between two α-helical clusters (Lawrence et al., 1990, 1994), one located near the center of the linear sequence and one located near the C terminus (Figures 1A and 1B). The crystal structure of phaseolin reveals that the C-terminal α-helical region of a monomer interacts with the central α-helical region of the interacting monomer to form trimers (Figures 1B and 1C).
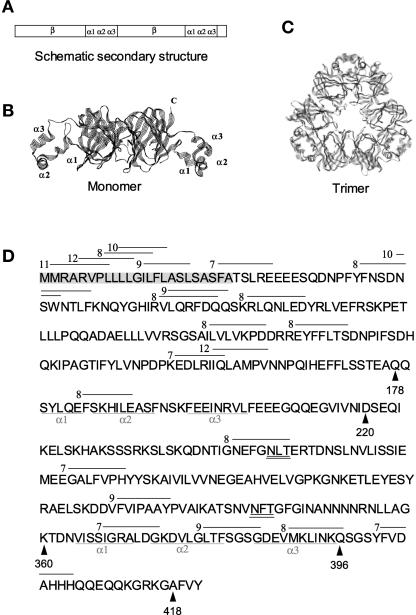
Figure 1.
Phaseolin Structure and Potential BiP Binding Sites.
(A) Scheme of the secondary structure of a phaseolin polypeptide. α1α2α3, α-helical clusters involved in assembly; β, β-barrel domains.
(B) Three-dimensional structure of a phaseolin polypeptide. N, N terminus; C, C terminus.
(C) Three-dimensional structure of a phaseolin trimer.
(D) Amino acid sequence of β-phaseolin as deduced from the cDNA sequence, including the signal peptide (highlighted in gray). The α-helical domains involved in subunit contacts are underlined. The lines above the sequence indicate the putative BiP binding sites, and the numbers indicate their BiP affinity scores according to the BiP Score algorithm. Only sites with a score unequivocally >6 are shown. The glycosylation sites are double underlined. The arrowheads point to residues relevant in the construction of the recombinant proteins used in this work.
The structures in (B) and (C) were drawn from file 2PHL.pdb using RasMol.
We have shown previously that phaseolin polypeptides associate transiently with BiP only before trimer formation (Vitale et al., 1995). Conversely, phaseolin mutants that are unable to form trimers show prolonged interactions with BiP in the ER before being degraded by quality control (Pedrazzini et al., 1994, 1997; Frigerio et al., 2001b). This stable interaction suggests that BiP binding sites normally hidden in the trimers remain exposed in assembly-defective monomers. Therefore, we resolved to determine whether phaseolin regions involved in trimerization are effectively BiP binding sites in vivo. The results presented here confirm this hypothesis.
RESULTS
The C-Terminal Domain of Phaseolin Stimulates the Interaction of Secretory Green Fluorescent Protein with BiP
We have shown previously that mutants of phaseolin partially or totally lacking the C-terminal domain are unable to assemble (Pedrazzini et al., 1994, 1997; Frigerio et al., 2001b). This domain includes a cluster of three α-helices followed by a less structured terminal sequence (Figure 1) (Lawrence et al., 1994). The last four amino acids (AFVY; Figure 1D) are a propeptide removed upon vacuolar delivery and constitute the vacuolar sorting signal of phaseolin (Frigerio et al., 1998). We wanted to determine whether the C-terminal domain contains sites that actually promote BiP binding in vivo. According to the BiP scoring algorithm, heptapeptides with scores >6 have a 50% or greater probability of binding to BiP in vitro (Knarr et al., 1999). The C-terminal domain of phaseolin is predicted by the program to contain 4 putative BiP binding sites with scores >6, out of 17 such sites in the sequence of mature phaseolin (Figure 1D). The signal peptide contains multiple additional sites (Figure 1D). This has been noted for other proteins (Blond-Elguindi et al., 1993; Knarr et al., 1999) and is expected, given the hydrophobic nature of signal peptides. Signal peptides bind the protein translocation channel of the ER (Mothes et al., 1998), and in most cases, including that of phaseolin (Bollini et al., 1983), they are cleaved off the nascent chain cotranslationally. However, it has not been established conclusively whether they ever come in contact with BiP.
To test the ability of the phaseolin C-terminal domain to promote interactions with BiP, we used a secretory form of green fluorescent protein (GFP) as a reporter. GFP is a small soluble protein that is secreted efficiently when introduced into the plant ER via a signal peptide (Batoko et al., 2000; Frigerio et al., 2001a). A number of C-terminal additions have been performed on ER-introduced GFP without affecting its fluorescence, and therefore its folding, in plant cells (Haseloff et al., 1997; Di Sansebastiano et al., 1998; Benghezal et al., 2000; Frigerio et al., 2001a). These characteristics make it a good reporter for studies of plant secretory pathway mechanisms. We first fused the phaseolin sequence encoding amino acids 361 to 417 in frame to the C terminus of a secreted form of GFP (sGFP; which contains a signal peptide for entry into the ER) in a construct termed sGFP418 (Figure 2). In sGFP418, the C-terminal domain of phaseolin should be forced permanently into a state similar to that in unassembled phaseolin subunits, because it cannot interact with its natural interchain partner. The phaseolin fragment in sGFP418 includes the C-terminal α-helical region but is devoid of the vacuolar sorting signal (Figures 1D and 2). This signal is not part of a strong potential BiP binding sequence and is not necessary for phaseolin trimerization, but it must promote uncharacterized interactions between phaseolin and the vacuolar sorting machinery, because a mutated phaseolin without AFVY forms trimers efficiently but is secreted (Frigerio et al., 1998). To simplify as much as possible our protein–protein interaction system, we avoided the inclusion of the AFVY sequence in the GFP construct.
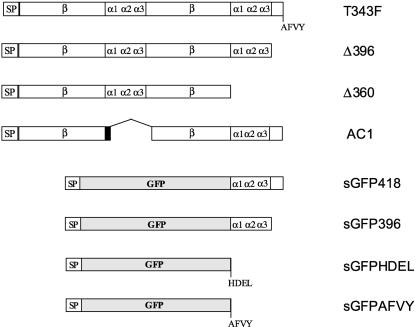
Figure 2.
Scheme of Polypeptides Encoded by the DNA Constructs Used in This Work.
All phaseolin constructs, except AC1, are derived from T343F and therefore have only one glycosylation site, at Asn-252. AC1 is derived from wild-type phaseolin and therefore also is glycosylated at Asn-341. When present, the phaseolin vacuolar sorting signal AFVY is indicated. The black portion of AC1 indicates the unstructured nine–amino acid linker added to this construct. α1α2α3, α-helical clusters involved in phaseolin assembly; β, β-barrel domains; SP, signal peptide.
We generated transgenic tobacco plants expressing sGFP418. To detect BiP interactions, protoplasts were isolated from leaves of transgenic plants and subjected to pulse labeling for 1 h using a mixture of 35S-Met and 35S-Cys. Protoplast homogenates then were immunoprecipitated with either anti-GFP or anti-BiP antiserum, and the selected polypeptides were analyzed by SDS-PAGE and fluorography. When anti-GFP was used for the first round of immunoprecipitation, a polypeptide of the size expected for sGFP418 was selected (Figure 3, lane 1). Upon longer film exposure, a polypeptide of the size predicted for BiP also was detectable (data not shown). When using anti-BiP antiserum, the major band coselected with BiP comigrated with sGFP418 (Figure 3, lane 2). In vitro treatment with ATP stimulates the ATPase activity of BiP, causing the release of the bound proteins or peptides (for phaseolin, see Vitale et al., 1995; Pedrazzini et al., 1997). ATP treatment in vitro fully released the putative sGFP418 from the anti-BiP immunoprecipitate, indicating that the association is attributable to the chaperone activity of BiP and not to nonspecific interactions (Figure 3, lane 3). This finding is similar to that observed for assembly-defective phaseolin mutants (Pedrazzini et al., 1997). The ATP-released material was recognized by anti-GFP antiserum (Figure 3, lane 4), confirming that sGFP418 is the major newly synthesized ligand of BiP in this transgenic plant. Analysis of protoplasts by confocal microscopy showed that sGFP418 is fluorescent (data not shown). Thus, we postulate that the fusion protein is folded correctly; therefore, BiP binding at sGFP418 sequences normally hidden in wild-type GFP is unlikely.
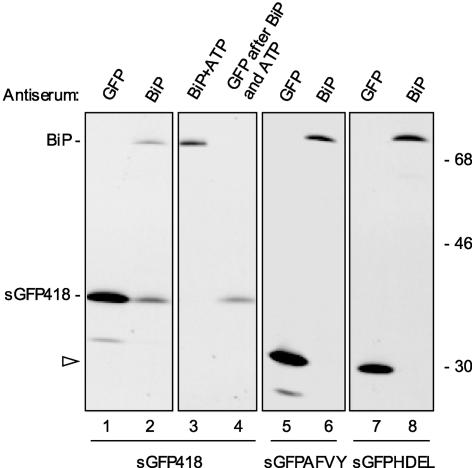
Figure 3.
The C-Terminal Portion of PHSL Promotes Binding of BiP to a Reporter Protein.
Protoplasts prepared from transgenic plants expressing sGFP418 (lanes 1 to 4), sGFPAFVY (lanes 5 and 6), or sGFPHDEL (lanes 7 and 8) were pulse-labeled for 1 h with 35S-Met and 35S-Cys. Cells then were homogenized and subjected to immunoprecipitation with either anti-GFP or anti-BiP antiserum, as indicated. The immunoselected material was analyzed by SDS-PAGE and fluorography. An aliquot of the material immunoselected with anti-BiP antiserum from plants expressing sGFP418 (lane 2) was treated in vitro with ATP (lane 3), and the released material was used for immunoselection with anti-GFP antiserum (lane 4). The positions of sGFP418 and BiP are indicated at left. The arrowhead at left points to the positions of sGFPAFVY (lane 5) and sGFPHDEL (lane 7), which have similar molecular masses. Numbers at right indicate molecular mass markers in kilodaltons.
As controls, we produced and analyzed transgenic tobacco plants expressing an HDEL-tagged version or an AFVY-tagged version of sGFP. Many soluble residents of the ER have the tetrapeptide HDEL or KDEL as C-terminal sequences. These tetrapeptides afford ER residence and function also when placed by genetic engineering at the C terminus of proteins normally destined to secretion or vacuolar delivery (for review, see Vitale and Denecke, 1999). Thus, sGFPHDEL is retained in the ER and is used to highlight this compartment in living plant cells (Haseloff et al., 1997). sGFPAFVY, on the other hand, is sorted to the vacuole (Frigerio et al., 2001a). After 1 h of pulse labeling, neither of the two GFP constructs could be coselected using anti-BiP antiserum (Figure 3, lanes 5 to 8), indicating that permanence in the ER does not result directly in the interaction with BiP detected by our assay and that the addition of the vacuolar sorting signal of phaseolin does not stimulate interactions with BiP.
Therefore, the portion of phaseolin fused to GFP in the construct sGFP418 is sufficient to stimulate BiP binding. We also produced a GFP fusion to a shorter version of the C-terminal domain of phaseolin that includes only the three α-helical segments (residues 361 to 395) (Figure 1D). This construct was termed sGFP396 (Figure 2). Some of the amino acids immediately following residue 396 are involved in intrachain interactions with the C-proximal β-sheet domain (Lawrence et al., 1994), possibly contributing to positioning the C-terminal helical cluster in the folded phaseolin monomer. The rest of the phaseolin region between amino acids 396 and 417 is mostly unstructured (Lawrence et al., 1990, 1994), but it contains one potential BiP binding site (Figure 1D). The interactions of BiP with sGFP418 and sGFP396 were compared by transient expression in tobacco protoplasts followed by radioactive pulse labeling. The anti-BiP antiserum coselected polypeptides of the molecular weights corresponding to each of the GFP fusions (Figure 4, lanes 1 to 4; cf. immunoselections with anti-GFP and anti-BiP antiserum for each construct). ATP treatment of the anti-BiP immunoprecipitate followed by immunoprecipitation of the released material with anti-GFP antiserum confirmed that sGFP396 was coselected with BiP (Figure 4, lanes 5 to 7). sGFP396 accounts for only a portion of the total amount of the fusion protein synthesized after a 1-h pulse, but it is a major component associated with BiP (Figure 4, lanes 1 and 2). In this respect, there does not seem to be a marked difference between it and sGFP418 (Figure 4, lanes 3 and 4). We conclude that the three C-terminal α-helical segments of phaseolin are sufficient to stimulate the association of BiP with the reporter protein.
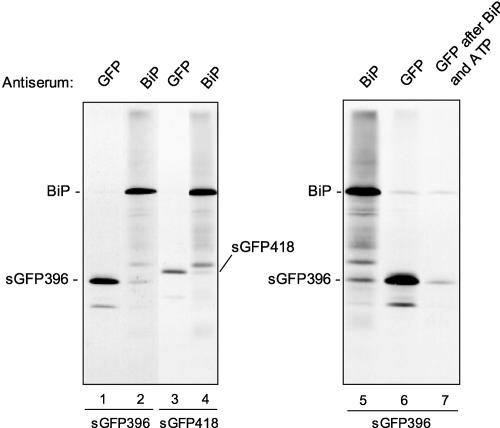
Figure 4.
The C-Terminal α-Helical Region of Phaseolin Is Sufficient to Promote BiP Binding to a Reporter Protein.
Tobacco leaf protoplasts were transfected transiently with plasmids encoding sGFP396 (lanes 1, 2, and 5 to 7) or sGFP418 (lanes 3 and 4) and pulse-labeled for 1 h with 35S-Met and 35S-Cys. Cell homogenates were subjected to immunoprecipitation with anti-BiP or anti-GFP antiserum as indicated. The sample in lane 7 was first immunoselected with anti-BiP antiserum and treated in vitro with ATP; the released material finally was used for immunoselection with anti-GFP antiserum. The positions of the GFP fusions and of BiP are indicated.
Note that the sGFP constructs used in this study also produce a minor polypeptide of lower molecular mass (visible in Figure 4, lanes 1 and 3; see also Figure 3, lanes 1 and 5; in the case of GFPHDEL—lane 7 in Figure 3—the fragment is below the portion of the fluorograph shown in the figure). The decrease in molecular mass is ∼4 kD and is independent of the length of the added phaseolin segment. Therefore, these fragments must be devoid of an N-terminal portion of GFP, but we do not know if they originate from alternative translation or from very rapid minor proteolysis. This question was not investigated further.
The C-Terminal Phaseolin α-Helical Domain Increases the Affinity of Unassembled Phaseolin Monomers for BiP
We next wanted to determine whether the C-terminal α-helical domain promotes the binding of phaseolin monomers to BiP. For this purpose, we needed to compare the behavior of phaseolin mutants that remain monomeric (because we know that assembly conceals BiP binding sites) with or without the C-terminal α-helical segments.
T343F phaseolin was the starting construct to produce the assembly-defective mutants Δ396 and Δ360 (Figure 2). T343F is assembly competent and sorted to the vacuole (Pedrazzini et al., 1997). In T343F (and all of its derivatives), the second glycosylation site, N341FT, is inactivated by mutagenesis (Pedrazzini et al., 1997). This site is used only partially in vivo (Bollini et al., 1983), and its inactivation does not affect the in vivo assembly or intracellular traffic of phaseolin, but it makes its banding pattern simpler (Pedrazzini et al., 1997; Frigerio et al., 1998, 2001b). Therefore, the phaseolin constructs derived from T343F have a single glycosylation site that is used efficiently, N252LT, although a small proportion of each construct also is synthesized in an unglycosylated form; on SDS-PAGE gels, each construct is represented by an abundant band and a second, less abundant (sometimes barely detectable) and slightly faster migrating band.
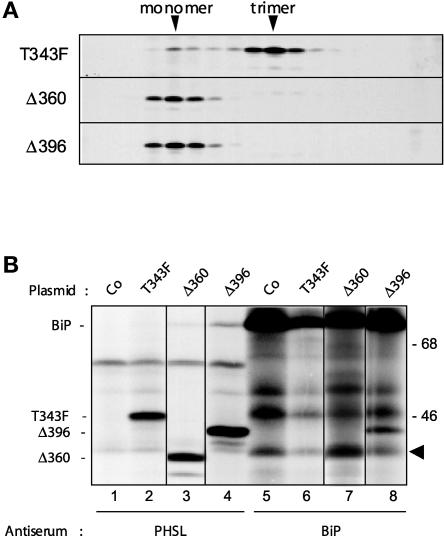
Phaseolin Δ360 has a stop codon before the start of the helical cluster (Figures 1D and 2) and remains monomeric in vivo (Pedrazzini et al., 1997). A new phaseolin mutant, Δ396, was produced; it has a stop codon immediately after the end of the third and last C-terminal α-helix (Figures 1D and 2). The portion present in Δ396 but not in Δ360 corresponds to the segment added to sGFP396. We first verified whether Δ396 is able to trimerize. Tobacco protoplasts were transfected transiently with plasmids encoding Δ396, Δ360, or T343F. After pulse labeling, cell homogenates were treated with ATP and subjected to centrifugation on a sedimentation velocity sucrose gradient. ATP treatment was performed to ensure that phaseolin monomers would not remain bound by BiP in this experiment. Proteins were immunoselected from each fraction with anti-phaseolin antiserum. Figure 5A shows that both Δ360 and Δ396 sediment as monomers, whereas the vast majority of T343F molecules were already trimeric after the pulse. Therefore, Δ396 is an assembly-defective mutant, like Δ360. This is probably because, as noted above, the sequence immediately following the third α-helix has intrachain interactions with the second β-sheet domain (Lawrence et al., 1994). Such interactions may play a key role in positioning the C-terminal helical cluster in the configuration needed for interchain contacts.
Figure 5.
Δ396 and Δ360 Are Assembly-Defective Mutants.
(A) Tobacco leaf protoplasts were transfected transiently with plasmids encoding T343F, Δ396, or Δ360 and pulse-labeled for 1 h with 35S-Met and 35S-Cys. Cell homogenates were treated with ATP to release BiP from its ligands and subjected to sedimentation velocity fractionation on a continuous sucrose gradient. Gradient fractions were subjected to immunoprecipitation with the anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. The top of the gradients is at left. The positions of monomers and trimers are indicated at top. Only the portions of the gels containing phaseolin are shown.
(B) Tobacco leaf protoplasts were transfected transiently with plasmids encoding the phaseolin constructs T343F, Δ396, and Δ360 or with the pDHA plasmid without insert (Co). Cells were pulse-labeled with 35S-Met and 35S-Cys for 1 h. Cells then were homogenized and subjected to immunoprecipitation with either anti-phaseolin or anti-BiP antiserum, and the immunoselected material was analyzed by SDS-PAGE and fluorography. The positions of BiP and of the phaseolin constructs are indicated at left. The arrowhead indicates an endogenous tobacco polypeptide that normally is coselected using anti-BiP antiserum but that is not related to phaseolin. Numbers at right indicate molecular mass markers in kilodaltons.
When immunoselection after pulse labeling was performed with anti-phaseolin antiserum, BiP was coselected with Δ396 and, to a lesser extent, with Δ360 (Figure 5B, cf. lanes 3 and 4). As expected, the association of BiP with T343F, which rapidly forms trimers, was barely detectable (Figure 5B, lane 2). Consistently, when immunoselection was performed with anti-BiP antiserum, Δ396 but not T343F was coselected with the chaperone (Figure 5B, cf. lane 6 with lane 8). The identification of Δ360 coselected using anti-BiP antiserum is problematic, because one of the endogenous tobacco polypeptides coselected with the chaperone (Figure 5B, arrowhead; see the control sample in lane 5) migrates just above the phaseolin mutant. However, it appears that Δ360 also is coselected with BiP, as expected, albeit to a lesser extent than Δ396 (Figure 5B, lane 7).
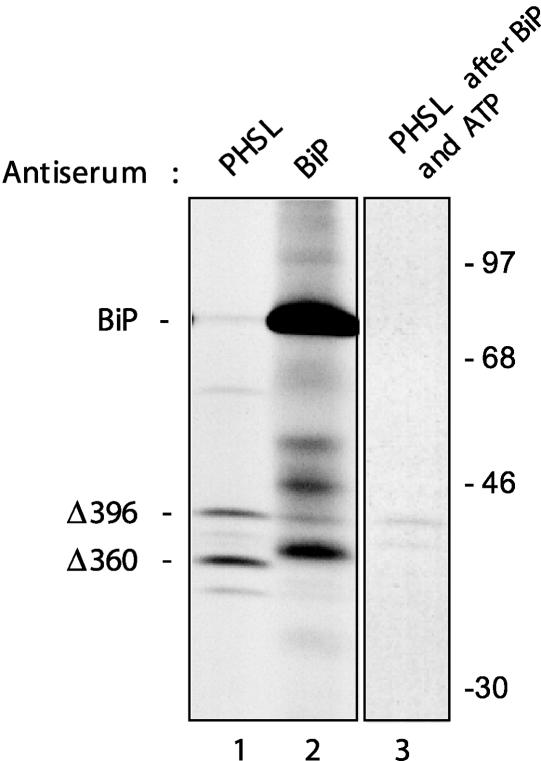
To directly compare the affinity of BiP for Δ360 and Δ396, the two phaseolin constructs were coexpressed in tobacco protoplasts. After pulse labeling, proteins were immunoselected with either anti-phaseolin or anti-BiP antiserum. Preliminary experiments (data not shown) were performed in which the two plasmids were mixed in different proportions to establish similar levels of synthesis of the two constructs. The results shown in Figure 6 indicate that even when Δ360 is synthesized in slightly greater amounts than Δ396 (lane 1), a larger proportion of the latter clearly was associated with BiP in an ATP-sensitive manner (lanes 2 and 3) (Δ360 associated with BiP is visible only after a longer exposure of the fluorograph; data not shown). This finding indicates that the C-terminal α-helical region of phaseolin promotes BiP binding to truncated, assembly-defective phaseolin and suggests that it also is a determinant for BiP binding to unassembled wild-type phaseolin.
Figure 6.
The C-Terminal α-Helical Domain Is a Determinant for BiP Binding to Phaseolin Monomers.
Tobacco leaf protoplasts were cotransfected transiently with plasmid encoding the phaseolin constructs Δ396 and Δ360. Cells were pulse-labeled with 35S-Met and 35S-Cys for 1 h. Cell homogenates then were subjected to immunoprecipitation with either anti-phaseolin or anti-BiP antiserum, and the immunoselected material was analyzed by SDS-PAGE and fluorography. An aliquot of the material immunoselected with anti-BiP antiserum (lane 2) was treated in vitro with ATP, and the released material was used for immunoselection with anti-PHSL antiserum (lane 3). Lane 3 shows a longer exposure compared with lanes 1 and 2. Numbers at right indicate molecular mass markers in kilodaltons.
Deletion of the Internal Region Involved in Contacts between Monomers Inhibits Assembly and Stimulates BiP Binding
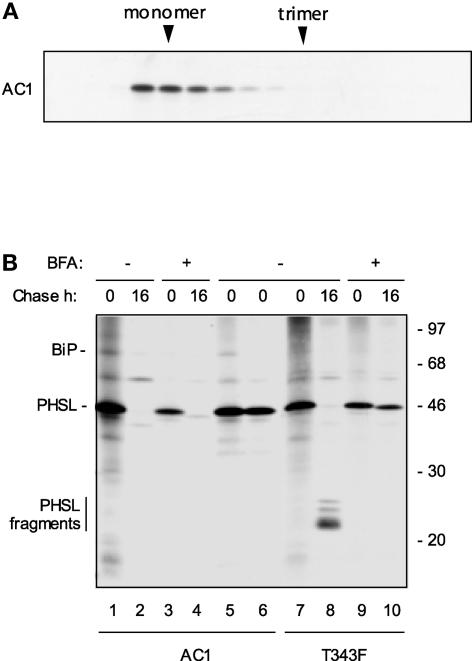
In addition to the C-terminal α-helical domain, other portions of phaseolin contain potential BiP binding sites (Figure 1D). One site is located within the internal α-helical region that interacts with the C-terminal α-helical region in phaseolin trimers (Figure 1). We tested the effects of deleting this region from phaseolin. The deletion involved amino acids 178 to 220. This segment is mainly α-helical but also contains a final portion with unordered structure (Lawrence et al., 1990). Val-217 and Ile-219 belong to this final portion and interact directly with the C-terminal helix 2 (Lawrence et al., 1994) (these two amino acids are numbered Val-193 and Ile-195 in that study because the phaseolin signal peptide was not included in the numeration; our numeration starts from the beginning of translation). We also introduced a linker of nine amino acids with unordered structure in an effort to disturb as little as possible the movement and folding of the portion of phaseolin following the deletion. This construct, termed AC1 (Figure 2), was expressed in transgenic tobacco under the control of the 35S promoter.
Protoplasts were prepared from leaves and subjected to radioactive labeling. Velocity centrifugation showed that AC1 does not assemble (Figure 7A). Pulse-chase analysis revealed that AC1 is degraded without the production of detectable fragments in a process that is largely insensitive to the vesicular traffic inhibitor brefeldin A (Figure 7B, lanes 1 to 4), similar to assembly-defective constructs lacking the C-terminal region (Pedrazzini et al., 1997). No secretion of AC1 was detectable (data not shown). Brefeldin A–insensitive degradation is diagnostic of the degradation of structurally defective proteins by ER quality control (for phaseolin, see Pedrazzini et al., 1997). A polypeptide corresponding to BiP was coselected with AC1 and released from the immunoprecipitate by ATP treatment (Figure 7B, lanes 1, 5, and 6). Figure 7B also shows the behavior of T343F in a similar pulse chase as a control (lanes 7 to 10). Upon vacuolar delivery in transgenic tobacco, phaseolin is hydrolyzed into fragments that can be used as markers for correct intracellular traffic (Sengupta-Gopalan et al., 1985; Pedrazzini et al., 1997). The fragmentation of T343F is inhibited by brefeldin A, because it requires traffic to the vacuole (Figure 7B, lanes 7 to 10) (Pedrazzini et al., 1997).
Figure 7.
AC1 Is Assembly Defective and Is Subject to ER Quality Control.
(A) Protoplasts from transgenic tobacco expressing AC1 were pulse-labeled for 1 h with 35S-Met and 35S-Cys. The cell homogenate was treated with ATP to release BiP from its ligands and subjected to sedimentation velocity fractionation on a continuous sucrose gradient. Gradient fractions were subjected to immunoprecipitation with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. The top of the gradient is at left. The positions of monomers and trimers are indicated at top. Only the portion of the gel containing phaseolin is shown.
(B) Protoplasts from transgenic tobacco expressing AC1 or T343F were pulse-labeled for 1 h with 35S-Met and 35S-Cys and chased for 0 or 16 h in the presence (+) or absence (−) of BFA. In lanes 1 to 4 and 7 to 10, cell homogenates were immunoprecipitated with anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. In lanes 5 and 6, pulse-labeled protoplasts from plants expressing AC1 were immunoprecipitated with anti-phaseolin antiserum; the immunoprecipitate was loaded either without further treatment (lane 5) or after treatment with ATP to release BiP (lane 6). The bar at left indicates the positions of the T343F fragmentation products. Numbers at right indicate molecular mass markers in kilodaltons.
We conclude that the internal region of phaseolin that is in contact with the C-terminal region is necessary for assembly and that its deletion promotes extended interactions with BiP followed by degradation by ER quality control.
Overexpressed BiP Transiently Sequesters Wild-Type Phaseolin
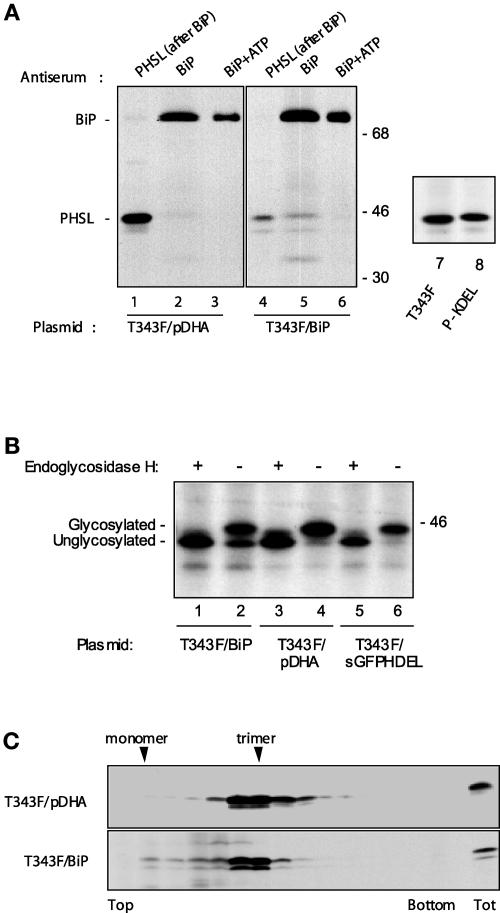
Our results indicate that BiP binds to at least one of the major regions involved in subunit interaction within the phaseolin trimer. Thus, we reason that in the ER there is competition between assembly and BiP association. This hypothesis was investigated by assessing whether overexpression of the chaperone could affect phaseolin trimerization. T343F phaseolin was coexpressed with a plasmid that encodes tobacco BiP under the control of the 35S promoter or with an equal amount of vector plasmid pDHA without insert as a control. As a first experiment, protoplasts were pulse-labeled for 1 h, homogenates were immunoselected using anti-BiP antiserum, and the unbound material was reimmunoselected with anti-phaseolin antiserum (Figure 8A). As expected, only a small proportion of T343F phaseolin was coselected with the chaperone in the control sample, whereas a large majority of the protein remained in the homogenate and was recovered only in the second round of immunoselection (Figure 8A, lanes 1 and 2). However, when BiP was overexpressed, a much larger proportion of newly synthesized phaseolin was found in association with the chaperone (Figure 8A, lanes 4 and 5). In vitro ATP treatment released this bound phaseolin (Figure 8A, lane 6), indicating that the association is caused by the chaperone activity of BiP. Thus, overexpression of BiP increased the proportion of T343F associated with the chaperone. Moreover, glycosylation of phaseolin was inhibited partially by BiP overexpression (Figure 8A, cf. the phaseolin banding patterns in lanes 1, 4, and 5), suggesting that BiP binding may compete with access to oligosaccharyl transferase and thus occurs, at least in part, cotranslationally.
Figure 8.
Overexpression of BiP Affects Phaseolin Maturation in the ER.
(A) In lanes 1 to 6, tobacco protoplasts were cotransfected transiently with the plasmid encoding T343F and the empty pDHA vector plasmid (lanes 1 to 3) or with plasmids encoding T343F and BiP (lanes 4 to 6). Cells were pulse-labeled for 1 h with 35S-Met and 35S-Cys. Cell homogenates then were subjected to immunoprecipitation with anti-BiP antiserum (lanes 2 and 5). The supernatants from these immunoprecipitations were reimmunoselected with anti-PHSL antiserum (lanes 1 and 4). An aliquot of the material immunoselected with anti-BiP antiserum was treated in vitro with ATP (lanes 3 and 6). An equal number of labeled protoplasts was used for immunoselection in each lane. Numbers at right indicate molecular mass markers in kilodaltons. In lanes 7 and 8, tobacco protoplasts were transformed transiently with plasmid encoding T343F or P-KDEL. Cells were pulse-labeled for 1 h with 35S-Met and 35S-Cys. Cell homogenates then were subjected to immunoprecipitation with anti-phaseolin antiserum. Only the portions of the gels containing phaseolin are shown.
(B) Tobacco protoplasts were cotransfected transiently with plasmids encoding T343F and BiP (lanes 1 and 2), T343F and empty pDHA plasmid (lanes 3 and 4), and T343F and sGFPHDEL (lanes 5 and 6). Cells were pulse-labeled for 1 h with 35S-Met and 35S-Cys. Proteins were immunoselected with anti-phaseolin antiserum and incubated with endoglycosidase H (+) or without the enzyme (−) as a control. Analysis was by SDS-PAGE and fluorography. Only the portion of the gel containing phaseolin is shown. The positions of glycosylated and unglycosylated phaseolin are indicated at left. The number at right indicates a molecular mass marker in kilodaltons.
(C) Tobacco protoplasts were cotransfected transiently with plasmid encoding T343F and empty pDHA plasmid or with plasmids encoding T343F and BiP. Cells were pulse-labeled for 1 h with 35S-Met and 35S-Cys. Cell homogenates then were treated with ATP and subjected to sedimentation velocity fractionation on a continuous sucrose gradient. Gradient fractions were subjected to immunoprecipitation with the anti-phaseolin antiserum and analyzed by SDS-PAGE and fluorography. The top of the gradients is at left. In each gel, the last lane at right (Tot) contains immunoprecipitate from total unfractionated homogenates corresponding to one-tenth of the material loaded on the gradient. Only the portions of the gels containing phaseolin are shown.
To verify that the faster migrating phaseolin band represents the unglycosylated polypeptide and not a product of proteolysis, phaseolin immunoprecipitated after pulse labeling was subjected to digestion in vitro by endoglycosidase H, an enzyme that removes N-linked glycans. Independently of BiP coexpression, digestion caused the disappearance of the slower migrating phaseolin band and its superimposition to the faster migrating band (Figure 8A, lanes 1 to 4). Most importantly, when BiP was coexpressed, no new band migrating faster than intact unglycosylated phaseolin was generated upon endoglycosidase H digestion (Figure 8A, lanes 1 and 2). This finding confirmed that no in vivo proteolysis occurred and that BiP coexpression inhibited phaseolin glycosylation.
Inhibition of glycosylation could be just a trivial effect of ER overload resulting from the transient expression of an ER-resident protein, in this case BiP. We have shown previously that P-KDEL (i.e., T343F with added KDEL at the C terminus) is a very stable protein that accumulates to high levels in the ER (Frigerio et al., 2001b). Therefore, the ER of protoplasts transfected with P-KDEL contained additional levels of resident protein probably comparable to those overexpressing BiP (labeling was performed ∼15 h after transfection). However, newly synthesized P-KDEL was glycosylated as efficiently as T343F (Figure 8A, lanes 7 and 8). In addition, when sGFPHDEL was coexpressed transiently with T343F, it did not inhibit phaseolin glycosylation (Figure 8B, lanes 5 and 6). These results indicate that the inhibition of phaseolin glycosylation that occurs upon BiP overexpression is not a side effect of nonspecific ER protein overload.
Given that in bean cotyledons, BiP interacts only with phaseolin monomers (Vitale et al., 1995), the results shown in Figure 8A suggest that overexpression of the chaperone inhibits trimer formation. Thus, we compared the assembly state of T343F with and without coexpression with BiP. After pulse labeling, cell homogenates were treated with ATP to destroy any interaction between BiP and phaseolin. Proteins then were separated by sedimentation velocity centrifugation on sucrose gradients, and phaseolin was immunoselected from each gradient fraction. The proportion of unassembled polypeptides clearly was increased upon BiP overexpression (Figure 8B).
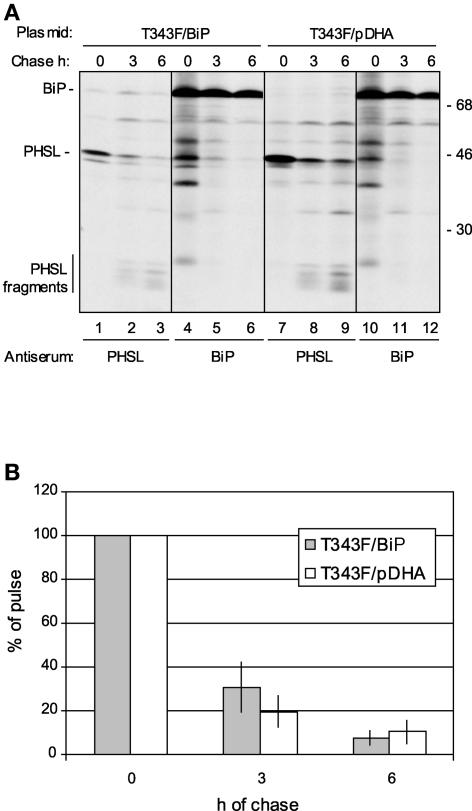
Finally, we wanted to determine whether BiP overexpression could influence the intracellular traffic of phaseolin along the secretory pathway. Pulse-chase labeling followed by immunoselection with anti-phaseolin or anti-BiP antiserum showed that under normal circumstances, T343F was released rapidly from the chaperone and recovered mostly as vacuolar fragments after a 6-h chase (Figure 9A, lanes 7 to 12). Upon BiP overexpression, a relevant proportion of intact phaseolin was associated with BiP during the chase; however, vacuolar fragments eventually were formed (Figure 9A, lanes 1 to 6). When immunoselection was performed with anti-BiP antiserum, phaseolin fragments never were coselected with the chaperone (Figure 9A, lanes 4 to 6 and 10 to 12), as expected from our previous observations that phaseolin intracellular traffic requires trimerization and is necessary for fragmentation (Pedrazzini et al., 1997).
Figure 9.
BiP Overexpression and Phaseolin Traffic.
(A) Tobacco protoplasts were cotransfected transiently with plasmid encoding T343F and empty pDHA plasmid or with plasmids encoding T343F and BiP. Cells were pulse-labeled for 1 h with 35S-Met and 35S-Cys and subjected to chase for 0, 3, or 6 h. Cell homogenates were immunoprecipitated with either anti-phaseolin or anti-BiP antiserum and analyzed by SDS-PAGE and fluorography. Numbers at right indicate molecular mass markers in kilodaltons.
(B) Experiments were performed as in (A). The relative intensities of the bands corresponding to intact phaseolin were measured by scanning densitometry of fluorographs. The values after the different chase times were calculated as percentages of the values at 0 h. The columns indicate the average values of four sets of immunoprecipitations from three fully independent protoplast preparations and transfections. Error bars indicate standard deviations.
On the whole, Figure 9A shows that association with overexpressed BiP can persist for a long time but is mostly a transient event that may slow, but not permanently block, phaseolin traffic. A direct measure of the rate and efficiency of vacuolar delivery is difficult in the case of transiently expressed phaseolin, because the high amount of protein synthesized per cell during transient expression saturates the vacuolar sorting machinery, leading to partial secretion (Frigerio et al., 1998). However, the time-dependent decrease in the recovery of intracellular intact phaseolin can provide an indication of the rate of export from the ER-Golgi system (with some limitations; see Discussion). Such quantitative analysis, performed by scanning of fluorographs, confirmed that BiP overexpression initially slowed but did not permanently inhibit phaseolin traffic (Figure 9B). Indeed, BiP overexpression may even enhance, albeit only slightly, the long-term efficiency of phaseolin traffic (Figure 9B, 6 h of chase).
DISCUSSION
Identification of a BiP Binding Region in Phaseolin
We have identified a relatively short sequence of phaseolin (57 amino acids) that promotes in vivo BiP binding when fused to the C terminus of a secretory version of GFP expressed in transgenic tobacco. The α-helical portion of this sequence (35 amino acids) also promotes BiP binding in transient expression experiments when fused to GFP and when present in assembly-defective phaseolin mutants. This portion is a major region of contact between subunits in wild-type phaseolin trimers. The 57–amino acid region contains four putative BiP binding sites, three of which are in the α-helical portion (Figure 1D), according to the score program defined by in vitro data obtained using synthetic peptides (Blond-Elguindi et al., 1993; Knarr et al., 1995, 1999). Site-directed mutagenesis will be necessary to verify whether the characteristics for BiP binding defined by in vitro experiments apply in vivo to this phaseolin region. Comparison of the in vivo association of BiP with sGFP396 and sGFP418 indicates that the sequence following the α-helical portion is not necessary for the association of BiP with the C-terminal segment of phaseolin. This finding supports a model in which reciprocal masking of the internal and C-terminal α-helical clusters is the determinant factor that conceals BiP interactions in phaseolin trimers in vivo.
Many newly synthesized secretory proteins have been found in vivo in transient association with BiP. However, in spite of the vast amount of information on in vitro recognition properties using synthetic peptides, studies of where BiP binds in vivo to a given protein are limited. To our knowledge, the more detailed in vivo studies concern immunoglobulin heavy and light chains and the L surface protein of hepatitis B virus (Hendershot et al., 1987; Skowronek et al., 1998; Cho et al., 2003). The identified BiP binding domains of these proteins are composed of ∼110 to 120 residues. Our study extends these findings to a shorter domain of a homotrimeric plant vacuolar protein.
Potential BiP binding sites are enriched in hydrophobic amino acids (Blond-Elguindi et al., 1993). Indeed, key interactions between phaseolin subunits, as well as in many other oligomeric proteins, involve hydrophobic contacts. It has been observed that among the amino acids involved in the assembly of phaseolin, Leu-380 and Phe-382 are conserved in 7S proteins and are replaced only by other hydrophobic residues (Lawrence et al., 1994; note that in that article, the two amino acids are designated Leu-356 and Phe-358, because, as noted above, the signal peptide of 24 amino acids was not considered). The two residues are part of a potential BiP binding site in phaseolin (Figure 1D). We cannot exclude the possibility that in Δ396 or sGFP396 the helical region containing the two residues has a different conformation than in assembled, wild-type phaseolin, but it is reasonable to assume that in the wild-type protein these residues interact with BiP until they are masked by trimerization, and our results support this hypothesis.
Certainly, the one we have identified is not the only in vivo BiP binding region of phaseolin, because it is absent in Δ360 and Δ363, two assembly-defective constructs that also interact with BiP (Pedrazzini et al., 1997). The BiP program also predicts 13 additional sites with scores unequivocally >6 (Figure 1D). Some of these sites may be concealed during polypeptide folding, whereas others can remain exposed until assembly. Indeed, one of the sites is part of the central region involved similarly in subunit contacts. We have shown here that deletion of this region inhibits phaseolin assembly in vivo. The mutated construct, AC1, interacts extensively with BiP and is subjected to ER quality control. Thus, it is tempting to speculate that in AC1 the C-terminal region remains permanently exposed to BiP interactions.
In our assay, sGFPAFVY does not associate with BiP to comparatively relevant levels, nor does sGFP tagged with the ER retention signal HDEL. These findings are consistent with our previous observation that the addition of KDEL to phaseolin confers ER residence, and therefore close proximity to the population of ER-resident chaperones (BiP included), but does not alter proper assembly and does not stimulate BiP to act as a chaperone on the recombinant protein (Frigerio et al., 2001b). Therefore, BiP can efficiently distinguish between unusually high concentrations of an H/KDEL-tagged but otherwise native protein in the plant ER and the presence of a sequence that has evolved to be masked during assembly processes.
BiP Binding and Phaseolin Maturation
We have shown here that BiP overexpression affects wild-type phaseolin. The proportion of BiP-associated polypeptides is increased, and newly synthesized monomers can be detected clearly. Finally, glycosylation is partially inhibited. We have shown that the inhibition of glycosylation is not a trivial side effect of the additional loading of the ER. Furthermore, tobacco BiP is not a glycoprotein; therefore, it cannot compete directly for glycosylation. We suggest that the decreased glycosylation is attributable to masking of the phaseolin glycosylation site (N252LT) by BiP binding; indeed, N252LT is part of a potential BiP binding site with a score of 8 (Figure 1D). Because the glycosylation of N252 is cotranslational (Bollini et al., 1983), this finding indicates that BiP binding to phaseolin could start cotranslationally, at least when the chaperone is overexpressed. Cotranslational association with BiP has been observed previously during the synthesis of rice prolamins (Li et al., 1993). Unlike phaseolin, these storage proteins assemble into extremely large and insoluble structures within the ER and are not transported farther.
The increased detection of phaseolin monomers when BiP is overexpressed is consistent with the finding that the C-terminal region involved in trimer formation also is a BiP binding determinant. Therefore, overexpressed BiP would interfere with the interactions between phaseolin monomers that lead to trimerization, possibly making more evident the competition between BiP binding and assembly that also could occur during the natural synthesis of phaseolin. Overexpressed BiP also could rescue newly synthesized subunits that normally aggregate or undergo other unproductive and nonspecific interactions before eventually being degraded by quality control. Judging from the proportion of newly synthesized phaseolin coselected with BiP when the chaperone is overexpressed, a sole rescuing function for the added BiP would indicate that unproductive phaseolin synthesis represents a relevant process in the absence of BiP overexpression, at least during transient synthesis in protoplasts. Moreover, it also suggests that the structurally unproductive phaseolin subunits become unavailable to immunoselection immediately after synthesis (perhaps because of aggregation and loss of solubility), whereas we have shown that severely defective phaseolin is available to immunoselection (AC1, Δ360, and Δ396 are discussed here; for Δ363, see Pedrazzini et al., 1997). Therefore, we favor the hypothesis that the main effect of the added BiP would be the transient inhibition of phaseolin assembly. However, the two roles of BiP—slowing assembly and preventing unproductive aggregation—are not necessarily alternative, as discussed briefly below.
BiP and the Intracellular Traffic of Phaseolin
Overexpression of BiP increases the production of functional recombinant proteins in yeast (Shusta et al., 1998) and insect cells (Tate et al., 1999) when measured as protein recovered after several days of culture. When the effect is measured using comparatively shorter pulse-chase experiments, overexpressed BiP was found to decrease the rates of structural maturation and secretion of a number of recombinant proteins (Dorner et al., 1992; Yang et al., 1998; Creemers et al., 2000; Jørgensen et al., 2000). The two effects are not in antithesis and could actually emphasize the nature of BiP as a chaperone. Chaperones are defined as proteins that increase the efficiency of correct structural maturation but actually decrease its rate. Quantitative analyses of our pulse-chase experiments are consistent with this scenario, because the sequestration of assembly-competent phaseolin by overexpressed BiP was not permanent and in the long term did not block vacuolar delivery of the storage protein. As noted above, we do not know the percentage of T343F phaseolin that fails to mature properly in our transient expression experiments, but we know that failure to trimerize results in degradation by ER quality control after prolonged ER retention and interactions with BiP (Pedrazzini et al., 1997). The availability of greater amounts of free BiP in the ER lumen may slow maturation but in the long term increase the probability of its final success if the bound ligands do not have permanent structural defects. It should be noted that this function of BiP is distinct from the one highlighted by Denecke and co-workers, who showed that BiP overexpression is necessary in tobacco protoplasts to support the synthesis of a secretory protein in the presence of ER stress (Leborgne-Castel et al., 1999). That function seems to be related to the translocation process, whereas the function highlighted here regards structural maturation. Together, these results emphasize the critical role of BiP in the synthesis of plant secretory proteins.
METHODS
Recombinant DNA
All DNA sequences were inserted under the control of the 35S promoter of Cauliflower mosaic virus in the expression vector pDHA. For Agrobacterium tumefaciens–mediated transformation of plants, the linearized expression vector containing the sequence of interest was inserted into the binary vector pGA470.
The phaseolin constructs T343F and Δ360 (formerly called T343FΔ360) have been described (Pedrazzini et al., 1997). The Δ396 deletion mutant was generated from the T343F coding sequence by introducing a stop codon at position 396 by means of m13-mediated mutagenesis. The following mutagenic antisense oligonucleotide was used: 5′-ACGATCCACTCTATTTGTTGATCAG-3′.
AC1 was constructed starting from the wild-type phaseolin cDNA clone β31 (Slightom et al., 1985). The sequence coding for amino acids 178 to 220 was deleted and substituted with a sequence coding for a nine–amino acid linker (GGGGSGGGG) using PCR-based mutagenesis and oligonucleotides 5′-TCGGATCCCGGGACTATGATGAGAGCAAGGGT-3′, 5′-TCGGATATAAGCTTGATATCGAATTC-3′, 5′-CCGGATCCGCCTCCACCGGCTTCTGTGCTAGA-3′, and 5′-GCGGATCCGGGGGTGGAGGTTCTGAACAGATTAAGGAAC-3′.
sGFPAFVY has been described (Frigerio et al., 2001a). sGFPHDEL (plasmid pBIN-mGFP5-ER; a gift from Jim Haseloff, University of Cambridge, UK) contains the GFP5 coding sequence fused to the Arabidopsis thaliana basic chitinase signal peptide and the C-terminal endoplasmic reticulum (ER) retrieval signal HDEL. To generate sGFP, the HDEL sequence was removed from the pBIN-mGFP5-ER construct by PCR amplification using primers 5′-GGATCCATGAAGACTAATCTTTTTCTC-3′ (forward) and 5′-CTGCAGTTATTTGTATAGTTCATCCAT-3′ (reverse). The amplified fragment was cloned into the BamHI-PstI sites of the expression vector pDHA. To generate sGFP418, the fusion between sGFP and residues 361 to 417 of phaseolin was obtained by PCR using the complementary oligonucleotides 5′-GGCATGGATGAAgcaggtaagacggacaatgtc-3′ and 5′-gacattgtccgtcttacctgcTTTGTATAGTTCATCCATGCC-3′, in which the bases shown in uppercase letters anneal to GFP and the bases shown in lowercase letters anneal to phaseolin. sGFP396 was generated by mutagenic PCR using sGFP418 as a template. The codon for Gln-396 (in the wild-type phaseolin sequence) was replaced by a stop codon using the mutagenic reverse primer 5′-CTGCAGCTATTTGTTGATCAGCTTCATAAC-3′. All constructs were confirmed by sequencing. The 35S-BiP overexpression construct was a gift from Jürgen Denecke (plasmid pDE800) (Leborgne-Castel et al., 1999).
Production of Transgenic Tobacco Plants and Transient Transformation of Leaf Protoplasts
A. tumefaciens LBA4404 containing sGFP418, sGFPHDEL, or AC1 was used to produce transgenic tobacco (Nicotiana tabacum cv Petit Havana SR1) plants as described by Pedrazzini et al. (1997). The production of transgenic tobacco expressing sGFPAFVY has been described (Frigerio et al., 2001a).
Protoplasts were prepared from axenic leaves (4 to 7 cm long) of tobacco cv Petit Havana SR1 (for transient transformation) or from transgenic tobacco plants. For transient transformation, protoplasts were subjected to polyethylene glycol–mediated transfection as described by Pedrazzini et al. (1997). Vector pDHA without inserts was used as a negative control for transfection. Forty micrograms of plasmid was used to transform protoplasts at a concentration of 106 cells/mL. After transfection, protoplasts were allowed to recover overnight in the dark at 25°C in K3 medium (Pedrazzini et al., 1997) at a concentration of 106 cells/mL before pulse-chase experiments were performed.
In Vivo Labeling of Protoplasts and Analysis of Proteins
Radioactive labeling of protoplasts was performed using Pro-Mix (a mixture of 35S-Met and 35S-Cys; Amersham Biosciences, Little Chalfont, UK) as described by Pedrazzini et al. (1997). Treatment with brefeldin A (Roche, Basel, Switzerland) was performed as described (Pedrazzini et al., 1997). Protoplast homogenization was performed by adding to frozen samples 2 volumes of ice-cold homogenization buffer (150 mM Tris-Cl, 150 mM NaCl, 1.5 mM EDTA, and 1.5% Triton X-100, pH 7.5) supplemented with Complete protease inhibitor cocktail (Roche).
Immunoselection was performed as described previously (D'Amico et al., 1992) using protein A–Sepharose and the following antisera: rabbit polyclonal antiserum raised against phaseolin purified from mature bean seeds (D'Amico et al., 1992); polyclonal anti-BiP antiserum raised against a recombinant fusion between maltose binding protein and amino acids 551 to 667 of tobacco BiP (Pedrazzini et al., 1997); and polyclonal antiserum against GFP (Molecular Probes, Eugene, OR).
For the analysis of phaseolin assembly by sedimentation velocity, after pulse labeling and homogenization, homogenates were brought to 8 mM MgCl2 and 3 mM ATP and loaded on top of a continuous 5 to 25% (w/v) linear sucrose gradient made in 150 mM NaCl, 1 mM EDTA, 0.1% Triton X-100, and 50 mM Tris-Cl, pH 7.5. Samples were centrifuged at 100,000g for 20 h at 20°C. Phaseolin then was immunoselected from each gradient fraction (usually 18 fractions per gradient, ∼700 μL each).
For digestion with endoglycosidase H, phaseolin was first immunoselected, and the protein A–Sepharose resin with the bound antibodies and antigen was washed twice with ice-cold water and resuspended in 50 μL of endoglycosidase H denaturing buffer (New England Biolabs, Beverly, MA). The resuspension was denatured for 10 min at 100°C. Five microliters of 10× G5 buffer (New England Biolabs) was added, and the solution was split into two aliquots. One aliquot (treated sample) was supplied with 200 International Union of Biochemistry milliunits of endoglycosidase H (recombinant; New England Biolabs); the other aliquot (control) was supplied with an equal volume of 20 mM Tris, 50 mM NaCl, 5 mM EDTA, pH 7.5 (this is the endoglycosidase H storage buffer). Incubation was for 1 h at 37°C. The samples then were subjected to SDS-PAGE.
Radioactive samples were analyzed by SDS-PAGE on 15% acrylamide gels. Rainbow 14C-methylated proteins (Amersham Biosciences) or protein molecular weight markers (Fermentas, Vilnius, Lithuania) were used as molecular weight markers. Radioactive polypeptides were revealed by fluorography. Gels usually were treated with 2,5-diphenyloxazole dissolved in DMSO and dried. Alternatively, gels were dried without treatment and exposed using the intensifying screen BioMax Transcreen LE (Kodak, Rochester, NY). Measurement of the relative intensities of the bands in the fluorographs was determined by microdensitometry. Care was taken to use film exposures that were in the linear range of film darkening.
Treatment with ATP
For ATP-mediated release of BiP, protoplasts were first homogenized and subjected to immunoselection using anti-BiP antiserum as described above. The protein A–Sepharose beads, washed three times with washing buffer (D'Amico et al., 1992), were incubated with 1 mL of BiP release buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 0.1% Triton X-100, 6 mM MgCl2, and 3 mM ATP) for 1 h at 4°C under gentle agitation. The material still bound to the beads after this treatment was analyzed by SDS-PAGE and fluorography. In some experiments, the ATP-released material was brought to 50 mM Tris-Cl, pH 7.5, and 0.25% gelatin and reimmunoselected using anti-GFP or anti-phaseolin antiserum.
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact A. Vitale, vitale@ibba.cnr.it.
Acknowledgments
We thank Andrea Pompa for skillful technical assistance. We also thank Aldo Ceriotti for preparing the AC1 construct. We are indebted to Jürgen Denecke (University of Leeds, UK) for the gift of the 35S-BiP overexpression construct and to Mary Jane Gething (University of Melbourne, Australia) for screening the phaseolin sequence for potential BiP binding sites using the BiP Score computer program. A grant from the British Council/Ministero dell'Università e della Ricerca Scientifica e Tecnologica (ROM/889/99/10) is gratefully acknowledged.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.013052.
References
- Augustine, J.G., de La Calle, A., Knarr, G., Buchner, J., and Frederick, C.A. (2001). The crystal structure of the Fab fragment of the monoclonal antibody MAK33: Implications for folding and interaction with the chaperone BiP. J. Biol. Chem. 276, 3287–3294. [DOI] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.Q., Hawes, C., and Moore, I. (2000). A rab1 GTPase is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plants. Plant Cell 12, 2201–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benghezal, M., Wasteneys, G.O., and Jones, D.A. (2000). The C-terminal dilysine motif confers endoplasmic reticulum localization to type I membrane proteins in plants. Plant Cell 12, 1179–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blond-Elguindi, S., Cwirla, S.E., Dower, W.J., Lipshutz, R.J., Sprang, S.R., Sambrook, J.F., and Gething, M.J. (1993). Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75, 717–728. [DOI] [PubMed] [Google Scholar]
- Bollini, R., Vitale, A., and Chrispeels, M.J. (1983). In vivo and in vitro processing of seed reserve protein in the endoplasmic reticulum: Evidence for two glycosylation steps. J. Cell Biol. 96, 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford, T.M., Gething, M.J., Davey, R., Hopwood, J.J., and Brooks, D.A. (1999). Processing of normal lysosomal and mutant N-acetylgalactosamine 4-sulphatase: BiP (immunoglobulin heavy-chain binding protein) may interact with critical protein contact sites. Biochem. J. 341, 193–201. [PMC free article] [PubMed] [Google Scholar]
- Cho, D.Y., Yang, G.H., Ryu, C.J., and Hong, H.J. (2003). Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo. J. Virol. 77, 2784–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers, J.W., van de Loo, J.W., Plets, E., Hendershot, L.M., and Van De Ven, W.J. (2000). Binding of BiP to the processing enzyme lymphoma proprotein convertase prevents aggregation, but slows down maturation. J. Biol. Chem. 275, 38842–38847. [DOI] [PubMed] [Google Scholar]
- D'Amico, L., Valsasina, B., Daminati, M.G., Fabbrini, M.S., Nitti, G., Bollini, R., Ceriotti, A., and Vitale, A. (1992). Bean homologs of the mammalian glucose regulated proteins: Induction by tunicamycin and interaction with newly synthesized storage proteins in the endoplasmic reticulum. Plant J. 2, 443–455. [DOI] [PubMed] [Google Scholar]
- Di Sansebastiano, G.P., Paris, N., Marc-Martin, S., and Neuhaus, J.M. (1998). Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J. 15, 449–457. [DOI] [PubMed] [Google Scholar]
- Dorner, A., Wasley, L.C., and Kaufman, R.J. (1992). Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J. 11, 1563–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, G.C., Pohl, J., Flocco, M.T., and Rothman, J.E. (1991). Peptide-binding specificity of the molecular chaperone BiP. Nature 353, 726–730. [DOI] [PubMed] [Google Scholar]
- Frigerio, L., de Virgilio, M., Prada, A., Faoro, F., and Vitale, A. (1998). Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10, 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., Foresti, O., Hernández Felipe, D., Neuhaus, J.-M., and Vitale, A. (2001. a). The C-terminal tetrapeptide of phaseolin is sufficient to target green fluorescent protein to the vacuole. J. Plant Physiol. 158, 499–503. [Google Scholar]
- Frigerio, L., Pastres, A., Prada, A., and Vitale, A. (2001. b). Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: Selective use of an alternative route to vacuoles. Plant Cell 13, 1109–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething, M.J. (1999). Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10, 465–472. [DOI] [PubMed] [Google Scholar]
- Haigh, N.G., and Johnson, A.E. (2002). A new role for BiP: Closing the aqueous translocon pore during protein integration into the ER membrane. J. Cell Biol. 156, 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamman, B.D., Hendershot, L.M., and Johnson, A.E. (1998). BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocon pore before and early in translocation. Cell 92, 747–758. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., Siemering, K.R., Prasher, D.C., and Hodge, S. (1997). Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94, 2122–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot, L., Bole, D., Kohler, G., and Kearney, J.F. (1987). Assembly and secretion of heavy chains that do not associate posttranslationally with immunoglobulin heavy chain-binding protein. J. Cell Biol. 104, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, M.M., Jensen, O.N., Holst, H.U., Hansen, J.J., Corydon, T.J., Bross, P., Bolund, L., and Gregersen, N. (2000). Grp78 is involved in retention of mutant low density lipoprotein receptor protein in the endoplasmic reticulum. J. Biol. Chem. 275, 33861–33868. [DOI] [PubMed] [Google Scholar]
- Knarr, G., Gething, M.J., Modrow, S., and Buchner, J. (1995). BiP binding sequences in antibodies. J. Biol. Chem. 270, 27589–27594. [DOI] [PubMed] [Google Scholar]
- Knarr, G., Modrow, S., Todd, A., Gething, M.J., and Buchner, J. (1999). BiP-binding sequences in HIV gp160: Implications for the binding specificity of BiP. J. Biol. Chem. 274, 29850–29857. [DOI] [PubMed] [Google Scholar]
- Lawrence, M.C., Izard, T., Beuchat, M., Blagrove, R.J., and Colman, P.M. (1994). Structure of phaseolin at 2.2 Å resolution: Implications for a common vicilin/legumin structure and the genetic engineering of seed storage proteins. J. Mol. Biol. 238, 748–776. [DOI] [PubMed] [Google Scholar]
- Lawrence, M.C., Suzuki, E., Varghese, J.N., Davis, P.C., Van Donkelaar, A., Tulloch, P.A., and Colman, P.M. (1990). The three-dimensional structure of the seed storage protein phaseolin at 3 Å resolution. EMBO J. 9, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leborgne-Castel, N., Jelitto-Van Dooren, E.P., Crofts, A.J., and Denecke, J. (1999). Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell 11, 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Wu, Y., Zhang, D.Z., Gillikin, J.W., Boston, R.S., Franceschi, V.R., and Okita, T.W. (1993). Rice prolamine protein body biogenesis: A BiP-mediated process. Science 262, 1054–1056. [DOI] [PubMed] [Google Scholar]
- Mothes, W., Jungnickel, B., Brunner, J., and Rapoport, T.A. (1998). Signal sequence recognition in cotranslational translocation by protein components of the endoplasmic reticulum membrane. J. Cell Biol. 142, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa, S.I., Fewell, S.W., Kato, Y., Brodsky, J.L., and Endo, T. (2001). Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J. Cell Biol. 153, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bielli, A., de Virgilio, M., Frigerio, L., Pesca, M., Faoro, F., Bollini, R., Ceriotti, A., and Vitale, A. (1997). Protein quality control along the route to the plant vacuole. Plant Cell 9, 1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bollini, R., Ceriotti, A., and Vitale, A. (1994). Binding of BiP to an assembly-defective protein in plant cells. Plant J. 5, 103–110. [Google Scholar]
- Sengupta-Gopalan, C., Reichert, N.A., Barker, R.F., and Hall, T.C. (1985). Developmentally regulated expression of the bean β-phaseolin gene in tobacco seeds. Proc. Natl. Acad. Sci. USA 82, 3320–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusta, E.V., Raines, R.T., Pluckthun, A., and Wittrup, K.D. (1998). Increasing the secretory capacity of Saccharomyces cerevisiae for production of single-chain antibody fragments. Nat. Biotechnol. 16, 773–777. [DOI] [PubMed] [Google Scholar]
- Skowronek, M.H., Hendershot, L.M., and Haas, I.G. (1998). The variable domain of nonassembled Ig light chains determines both their half-life and binding to the chaperone BiP. Proc. Natl. Acad. Sci. USA 95, 1574–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom, J.L., Drong, R.F., Klassy, R.C., and Hoffman, L.M. (1985). Nucleotide sequences from phaseolin cDNA clones: The major storage proteins from Phaseolus vulgaris are encoded by two unique gene families. Nucleic Acids Res. 13, 6483–6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparvoli, F., Faoro, F., Daminati, M.G., Ceriotti, A., and Bollini, R. (2000). Misfolding and aggregation of vacuolar glycoproteins in plant cells. Plant J. 24, 825–836. [DOI] [PubMed] [Google Scholar]
- Tate, C.G., Whiteley, E., and Betenbaugh, M.J. (1999). Molecular chaperones stimulate the functional expression of the cocaine-sensitive serotonin transporter. J. Biol. Chem. 274, 17551–17558. [DOI] [PubMed] [Google Scholar]
- Vitale, A., Bielli, A., and Ceriotti, A. (1995). The binding protein associates with monomeric phaseolin. Plant Physiol. 107, 1411–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Denecke, J. (1999). The endoplasmic reticulum: Gateway of the secretory pathway. Plant Cell 11, 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y., Turner, R.S., and Gaut, J.R. (1998). The chaperone BiP/GRP78 binds to amyloid precursor protein and decreases A40 and A42 secretion. J. Biol. Chem. 273, 25552–25555. [DOI] [PubMed] [Google Scholar]
- Zhu, X., Zhao, X., Burkholder, W.F., Gragerov, A., Ogata, C.M., Gottesman, M.E., and Hendrickson, W.A. (1996). Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272, 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]