Abstract
Axonal projections from the lateral superior olivary nuclei (LSO), as well as from the dorsal cochlear nucleus (DCN) and dorsal nucleus of the lateral lemniscus (DNLL), converge in frequency-ordered layers in the central nucleus of the inferior colliculus (IC) where they distribute among different synaptic compartments. A carbocyanine dye, DiI, was used as a tracer to study the postnatal development of axonal projections in the ferret IC. The results indicated that projections from all three nuclei are present at birth, but are not segregated into bands. During the postnatal week between approximately postnatal days 4 and 12 (P4-P12), axons from LSO proliferate in IC, become more branched, and segregate into a series of bands composed of densely packed fibers and endings. LSO projections in these afferent bands course parallel to IC layers and are separated by intervening regions with few endings. A modest fit of a sine curve (R2 >0.15) to the pattern of spacing of LSO projections in IC indicated that regularly spaced bands are forming by P7. Similarly, banded patterns of DCN and DNLL projections to IC have developed by the end of the first postnatal week. Thus, well before hearing onset in ferret (P28–30), three different afferent projections have segregated into banded compartments along layers in the central nucleus of the ferret IC. Possible mechanisms in circuit development are discussed.
Keywords: dorsal nucleus of the lateral lemniscus, lateral superior olivary nucleus, dorsal cochlear nucleus, fibrodendritic layers, postnatal, carbocyanine dye, DiI
INTRODUCTION
Fibrodendritic layers are characteristic of the architecture of input-output circuits in the central nucleus of the IC (e.g., Rockel and Jones, 1973; Oliver and Morest, 1984). Multiple ascending inputs to these layers from frequency-matched regions of lower brainstem auditory nuclei establish a cochleotopic order (e.g., Merzenich and Reid, 1974; Fitzpatrick, 1975; Roth et al., 1978; Kelly et al., 1998). It is clear from the diverse response characteristics of cells receiving this layered input (Semple and Aitkin, 1979; Wenstrup et al., 1986; Shreiner and Langner, 1988; Brückner and Rübsamen, 1995) that different synaptic compartments exist within a layer and integrate specific sets of inputs (Oliver and Shneiderman, 1989). In particular, axonal projections from the lateral superior olivary nuclei (LSO), the dorsal cochlear nucleus (DCN), and the dorsal nucleus of the lateral lemniscus (DNLL) all have been described as ending in a pattern of complementary bands and patches along layers of the IC (Shneiderman and Henkel, 1987; Shneiderman et al., 1988; Oliver et al., 1997) providing opportunities for synaptic interaction.
Initially response patterns of IC cells suggest that some components of this complex circuitry are relatively mature at hearing onset in kittens while others continue to be refined after hearing onset (Aitkin and Moore, 1975; Moore and Irvine, 1980, 1981). However, relatively little detailed information is known about the spatiotemporal sequence for assembly and refinement of complex synaptic circuits in IC layers. Previously, it has been shown that LSO bands in IC already are present and well refined by birth in the kittens (Brunso-Bechtold and Henkel, 2005; Henkel et al., 2005). Axonal growth, proliferation, and branching also have been carefully described for development of DCN projections to the rat IC (Kandler and Friauf, 1993), but patterns of banded afferent compartments in IC layers were not described in that study. On the other hand, a similar sequence of developmental events has been shown for DNLL projections to IC in rat (Gabriele et al., 2000a) and in that circuit axons are present at birth, but do not segregate into distinct bands in IC layers until postnatal day 8 (P8), four days before hearing onset (Jewett and Romano, 1972).
In the present study, we describe the development of axonal projections from LSO to IC and compare the developing LSO projection pattern with that from another lower brainstem auditory nucleus, DCN, and a higher auditory nucleus, DNLL. The goal was to determine when afferent projections segregate into banded compartments in IC in the ferret, an altricial carnivore with a more protracted postnatal period before hearing onset at P28-P30 (Moore, 1982). A carbocyanine dye, DiI, was placed in the nuclei or in their output tracts in postnatal ferrets to label their projections to the IC. We examined labeled projections from these nuclei at semi-weekly or weekly time points between P0 (birth) and P28. The results show that axonal projections from LSO, DCN and DNLL are present in ferret IC at birth and that banded projections from all three nuclei are present in IC two weeks prior to hearing onset. Thus, the timing of segregation of afferent bands from multiple sources is similar and precedes hearing onset.
Preliminary data on development of LSO projections to the ferret IC have been reported previously in abstract form (Keiger et al., 2005)
EXPERIMENTAL PROCEDURES
Axonal projections from brainstem auditory nuclei to the IC were studied in a collection of postnatal brains from 45 ferret kits labeled with a carbocyanine dye, 1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen-Molecular Probes, Eugene, OR, #22885). DiI is lipophilic and diffuses retrogradely in membranes back to the somata of labeled axons as well as anterogradely through the axolemma to label their branches and endings. All procedures complied with the Guide for the Care and Use of Laboratory Animals. Animals were housed and cared for in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). The experimental protocol was approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine.
DiI labeling and histology
Ferret kits were culled from the litter for study at postnatal ages P0, P4, P7–8, P13–14, P21–22, and P26–30. The kits were given an overdose of ketamine and xylazine and perfused through the heart with 4% paraformaldehyde. Afterward, the brain was removed, postfixed overnight in 4% paraformaldehyde, and blocked coronally to include the region from IC to DCN. A glass pin coated with DiI was inserted in one of the three brainstem target structures or in the IC. For pin placement in LSO or the DNLL commissure (Gabriele et al., 2000a), the tissue was embedded in egg yolk-gelatin and vibratome sectioned (St. Louis, MO) from either the caudal or rostral face of the embedded block until the target for DiI placement was visualized. After the DiI-pin was secure, the blocks were rinsed and returned to 4% paraformaldehyde for the duration of the incubation. DiI-labeled blocks were incubated in the dark at 37°C for 6 weeks to allow diffusion of the dye.
In some cases, DiI labeling of LSO projections to the contralateral IC was less extensive than labeling in the ipsilateral IC. Extending incubation times beyond 6 weeks appeared to have no consistent effect on the degree of labeling although this parameter can be important for visualizing projections (Lukas et al., 1998; Sparks et al., 2000). Perhaps myelination is more advanced for projections to the contralateral IC than for ipsilaterally projecting axons (e.g., sequential myelination of corpus callosum, Looney and Elberger, 1986; differential myelination of spinal tracts, Hamano et al., 1998) and, thus, interferes with DiI confusion or detection. The earlier birth dates of contralaterally projecting LSO cells in comparison with ipsilaterally projecting cells (Kudo et al., 1996) suggest that contralateral axon development could precede that for ipsilateral projections. Nevertheless, in those cases with well labeled contralateral and ipsilateral LSO fibers the patterns of distribution were not different and conclusions are based on those cases.
After incubation, 75μm thick coronal vibratome sections were cut through the brainstem. Sections were collected in phosphate buffer (0.1M, pH 7.2), mounted onto glass slides, and coverslipped with Gel/Mount (Biomeda, Foster City, CA, Catalogue #M01).
Image acquisition and processing
Sections were viewed with an Olympus BX2 epifluorescent photomicroscope (Melville, NY) equipped with a Cy5 filter set (Chroma Technologies, Brattleboro, VT) to visualize DiI labeled cells and axons. Digital images (1600x1200 dpi and 0–255 gray levels) were captured from the microscope using an Optronics Magnafire camera (Optronics, Goleta, CA). Images were collected in each case for the DiI pin site, labeled regions in brainstem auditory nuclei, and labeling in IC. Image processing software was used only to subtract background and to balance brightness and contrast. The final plates were composed and edited in Photoshop (Adobe Systems, San Jose, CA).
To analyze the banded organization of projections in selected cases, a rectangular region of interest was selected to encompass the dense axonal labeling in the central nucleus of the IC with the major axis of the rectangle oriented orthogonal to axonal layers in IC. The average line plot profile of image density along scan lines across this rectangle (orthogonal to the labeled axons) was then determined with ImageJ software (NIH, Bethesda, MD). Nonlinear regression (GraphPad Prism, San Diego, CA) was used to fit the density profile from labeled axonal plexuses in IC to a sine curve. A sine curve was chosen to model regular spacing of dense afferent bands across a series of layers (Gabriele et al., 2000b; Henkel et al., 2003; Henkel et al., 2005). At the outset, the center-to-center spacing (or period) in afferent banding patterns was measured directly from several cases. The median period subsequently was used in all cases to set the initial frequency value for curve fitting. Fit of the density data to a sine curve with an R2 value greater than 0.33 was considered to be a good fit and strong evidence of segregation into regularly spaced banded compartments.
RESULTS
Retrograde labeling sources of IC afferent projections
DiI-pins were placed in IC (Figure 1A) to label retrogradely axonal projections to IC and their cells of origin. The labeling pattern of one P7 case (Figure 1) that was illustrative of both the trajectory and origin of afferent projections to the IC at this time point will be described. Retrogradely labeled cells were most prominent in the contralateral DNLL, the contralateral and ipsilateral LSO, and the contralateral DCN (Figure 1B, C, and D). Although there were some labeled cells in other brainstem regions, (e.g., medial superior olivary nucleus, ventral nucleus of the lateral lemniscus, and ventral cochlear nucleus) these are not illustrated here.
Figure 1.
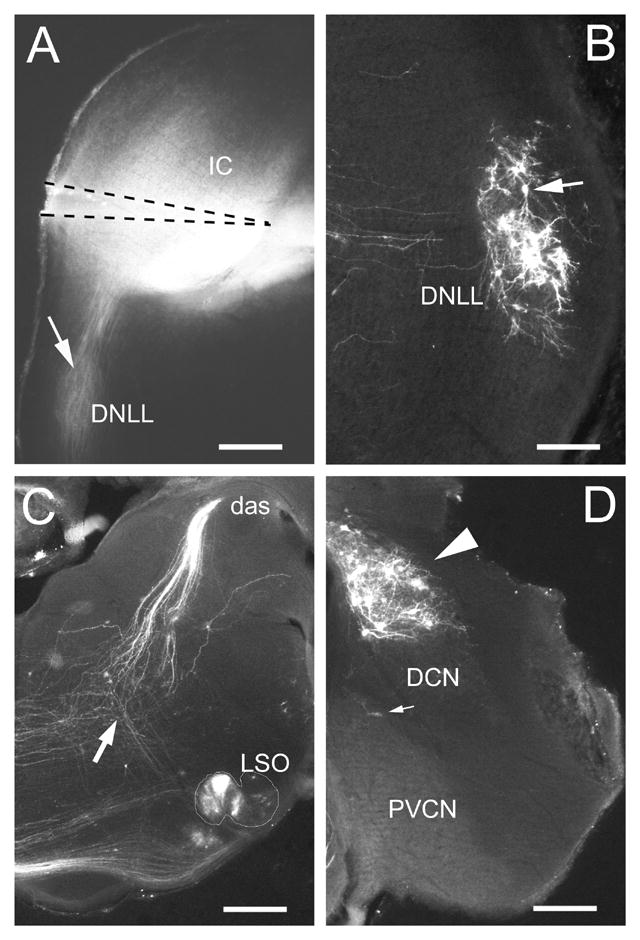
A. Epifluorescent image of coronal section through midlevel of IC from P7 ferret kit showing position of DiI-pin. Note that the brightest DiI-labeling filled the ventromedial part of the central nucleus of the IC. Retrogradely labeled fibers are visible in the ipsilateral DNLL and obscure lightly labeled cells (arrow) B. Retrogradely labeled cells (arrow) in the contralateral DNLL. C. Retrogradely labeled cells in the lateral superior olivary nucleus (LSO) (nuclear boundary is outlined) and fibers in the dorsal acoustic stria (das). An arrow indicates the point at which fibers from LSO cells converge with the dorsal acoustic stria in their course to the IC. D. Retrogradely labeled cells (arrowhead) in the contralateral dorsal cochlear nucleus (DCN). Few retrogradely labeled cells (small arrow) are observed in this portion of the posteroventral cochlear nucleus (PVCN). A, C – calibration bars equal 400μm; B, D – calibration bars equal 200μm.
Cells in the contralateral DNLL gave rise to a major projection to the IC and were heavily labeled (Figure 1B). Axons decussating in the dorsal tegmental commissure (of Probst) were traced from the DiI-pin site to their cells of origin in the DNLL. Although a few labeled cells could be detected in the ipsilateral DNLL, for the most part they were lightly labeled (arrow, Figure 1A) and obscured by labeling of the lateral lemniscal fibers.
In LSO, bilateral clusters of labeled cells were most dense medially, becoming sparser in the lateral portions of the nucleus (Figure 1C). Similarly, DiI label was denser in the ventral part of the medial superior olivary nucleus. Labeled cells were present in the periolivary cell groups but will not be considered here. Retrogradely labeled fibers could be followed to the LSO from the decussation in the same region as the dorsal acoustic stria (arrow in Figure 1C).
In the cochlear nuclear complex, labeled cells were located in the contralateral DCN (Figure 1D) and the posteroventral and caudal part of the anteroventral cochlear nuclei. In DCN, retrogradely labeled axons could be seen extending from their cells of origin to enter the dorsal acoustic stria (Figure 1C). The course and pontine decussation of the dorsal acoustic stria was clearly labeled in this case. Labeled fibers could also be followed in their course from the ventral cochlear nucleus to the ventral acoustic striae.
Anterograde labeling of axonal projections in IC
Retrograde labeling confirmed the presence of afferent axons in the IC in neonatal ferret kits, but did not permit the analysis of axonal distribution necessary to characterize the development of afferent bands in the ferret IC. For this purpose, we turned to results from cases that labeled axons anterogradely from each of three main sources: LSO, DCN, and DNLL. As a template for the temporal development of afferent bands in IC, LSO projections will be described first and then compared to projections from the other nuclei. DiI-pins in LSO and DCN in most cases produced bright epifluorescence outside of these nuclei. Thus, it is likely that some DiI spread to surrounding cell groups and/or auditory fiber tracts and may contribute to the labeling. Nevertheless, the patterns of axonal distribution in the central nucleus of the IC were consistent among all of the cases.
Development of afferent bands from LSO to IC
DiI-pins in LSO labeled axons in the ipsilateral and contralateral IC in P0, P4, P7, P14, P21/22, and P26/30 cases. Although the pattern of axon distribution was similar in the central nucleus of the ipsilateral and contralateral IC in most cases, labeling in the contralateral projection often appeared incomplete, presumably due to technical issues such as the longer distance for contralateral diffusion of the dye. A P7 case in Figure 2 illustrates the distribution of LSO axons in IC when projections to both sides were labeled (Figure 2, compare A and B). Although there are fewer labeled fibers in the contralateral IC, the general distribution of labeled axons was symmetric with that on the ipsilateral side. All LSO cases with relatively complete bilateral labeling revealed similar patterns of axon distribution on the ipsilateral and contralateral sides.
Figure 2.
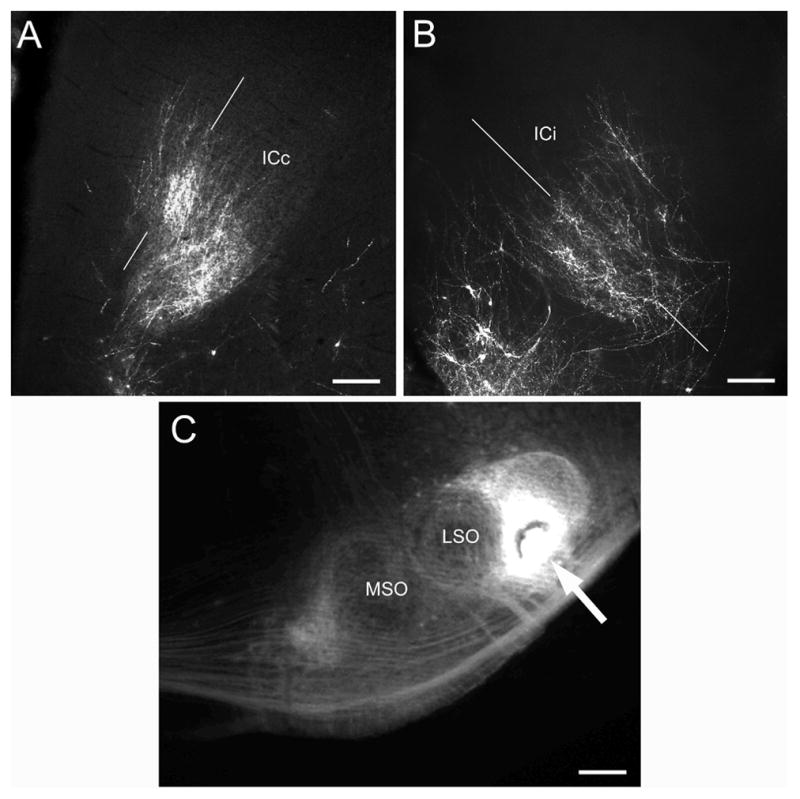
Epifluorescent images comparing the pattern of distribution of anterogradely labeled axons in the A) contralateral and B) ipsilateral IC in a P7 case with a C) DiI pin site (arrow) in the lateral superior olivary nucleus (LSO). Thin white lines in A and B indicate plane of developing bands. Other abbreviations: ICc, contralateral inferior colliculus; ICi, ipsilateral inferior colliculus; MSO, medial superior olivary nucleus. A, B – calibration bars equal 200μm; C – calibration bar equals 400μm.
In P0 and P4 cases, labeled fibers distributed diffusely in IC and most branches were oriented parallel to the plane of fibrodendritic layers in IC (Figure 3A). At more caudal levels in IC there was a paucity of labeled fibers and the few fibers that were present had relatively few tertiary branches (not shown). In the portions of the IC with the densest labeling, analysis of line plots of labeling density across axonal layers indicated a poor fit to a sine curve in cases from P0 and P4 LSO pin placements. Thus, there was little indication of band formation at these ages relative to older animals (compare Figure 3B, D, F).
Figure 3.
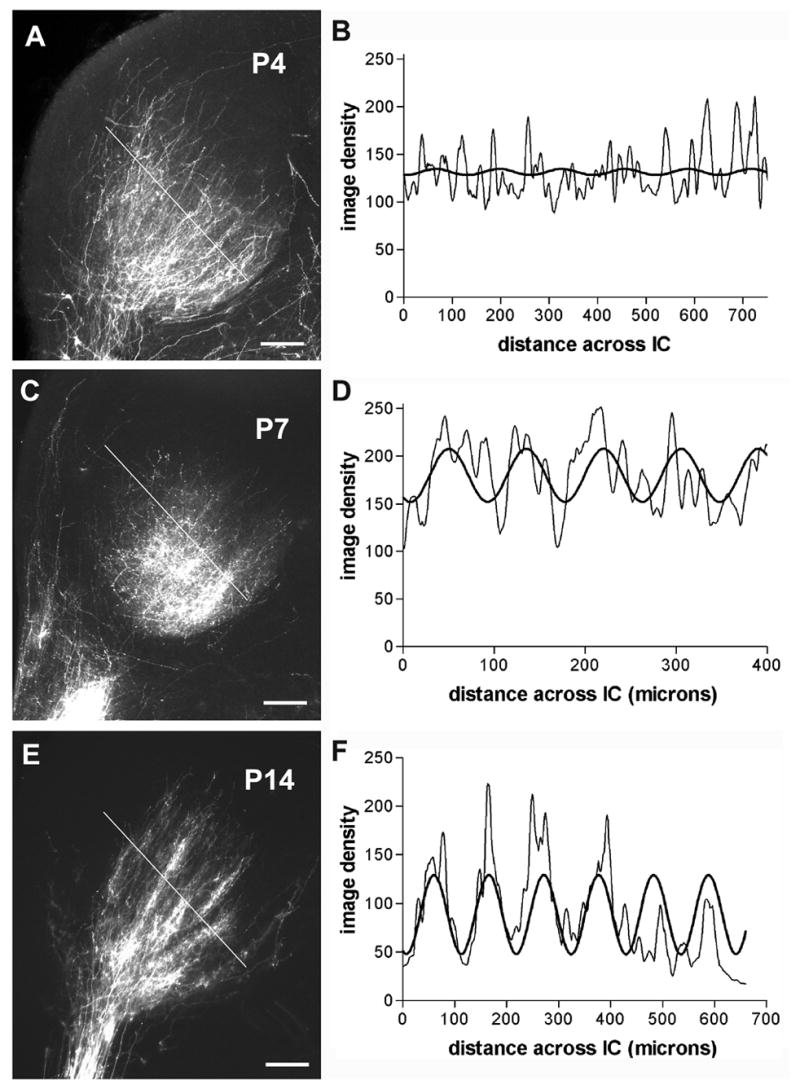
Epifluorescent images (left column) of DiI labeled LSO projections to IC with corresponding graphs (right column) showing line profiles of image density (thin graph line) orthogonal to plane of axonal layers and best fit sine curves (thick graph line). Dashed lines in A, C, and E indicate the orientation of selected field across IC labeling for density analyses shown in B, D, and F, respectively. Developmental series shows gradual segregation of banded afferent compartments (A, B examples from P4; C, D examples from P7; E, F examples from P14 ferret). A, C, E – calibration bars equal 200μm.
At P7, labeled fibers from LSO filled the central nucleus of the IC (Figure 3C). While fibers were distributed throughout IC, fibers oriented parallel to each other and to fibrodendritic layers were more apparent rostrally than caudally in IC. At this age a periodicity of labeling suggestive of the beginning of bands of LSO projections to IC could be distinguished. This variation was correlated with periodic waves in line density plots through the labeled region (Figure 3D). A sine curve fit the data better at this age (R2 ≥ 0.25) than for earlier ages.
Not until P14 were distinct bands of fibers anterogradely labeled from LSO clearly evident in the IC (Figure 3E). At P14, labeled bands consisted of axon branches and endings that were separated by intervening regions with only occasional labeled profiles. Often 5 or more distinct bands were apparent in a section. Measured as the center-to-center distance between brightly labeled bands, afferent bands were spaced 90–110μm apart. The dense bands of labeling and sparsely labeled interband spaces were similar in thickness. In the coronal plane, individual dense afferent bands were 450–950μm long. Line plots of labeling density orthogonal to the layers in P14 cases exhibited a regular periodicity of density peaks (Figure 3F). A good fit of the labeling density with a sine curve (R2>0.3) indicated marked delineation of banded afferent compartments by the end of the second postnatal week. The period of the modeled best fit sine curves was similar to actual spacing of the afferent bands measured from histologic sections (Figure 3E).
P21/22 and P26/28 cases showed no obvious changes in the general distribution pattern of labeled LSO fibers in IC from that seen at P14. However, these cases could not be analyzed further because the progressive increase in myelination at these ages interfered with the overall quality of DiI axonal labeling.
Development of afferent bands from DCN and DNLL to IC
Analysis of cases with DiI pins in the LSO indicated that LSO projections formed a banded pattern of in IC between P7 and P14. DCN and DNLL projections were examined at weekly postnatal intervals for comparison. Of particular interest was whether or not the time course of band formation for DCN and DNLL fibers within the IC was similar to that for LSO projections.
DiI-pins in DCN labeled considerably more fibers in the contralateral than ipsilateral IC (Figure 4). In P4 cases, branches of labeled DCN fibers were mostly linear in morphology and sparsely distributed parallel to layers in the contralateral IC (Figure 4B, see inset). Nonlinear regression showed only a poor fit of the data with a sine curve (Figure 5A, R2<0.1). The frequencies of these curves, however, were higher than in older animals indicating shorter distances between parallel axons that had not yet formed dense plexuses (compare Figures 5B and 3B). By P7, and certainly by P14, labeled fibers were more branched and distributed endings in dense plexuses (Figure 4C, D). Nonlinear regression showed good fit of a sine curve to the line plot of image density across the labeled DCN projections (Figure 5B, R2 ≥ 0.25).
Figure 4.
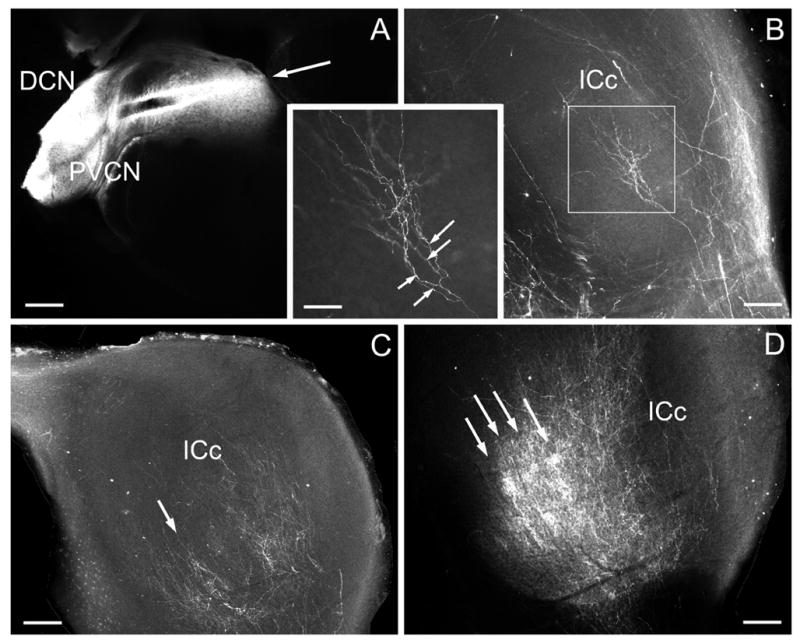
Epifluorescent image showing example of DiI-pin (arrow) in the DCN in P7 ferret (A) and labeled afferent fibers ending in the contralateral IC (ICc) for a P4 (B) , P7 (C), and P14 (D) case. Boxed area in B is inset showing arborization (arrows) of cochlear nucleus fiber. Regions of dense banded labeling are indicated by arrows in C and D. A-D – calibration bars equal 200μm; inset – calibration bar equal 80 μm.
Figure 5.
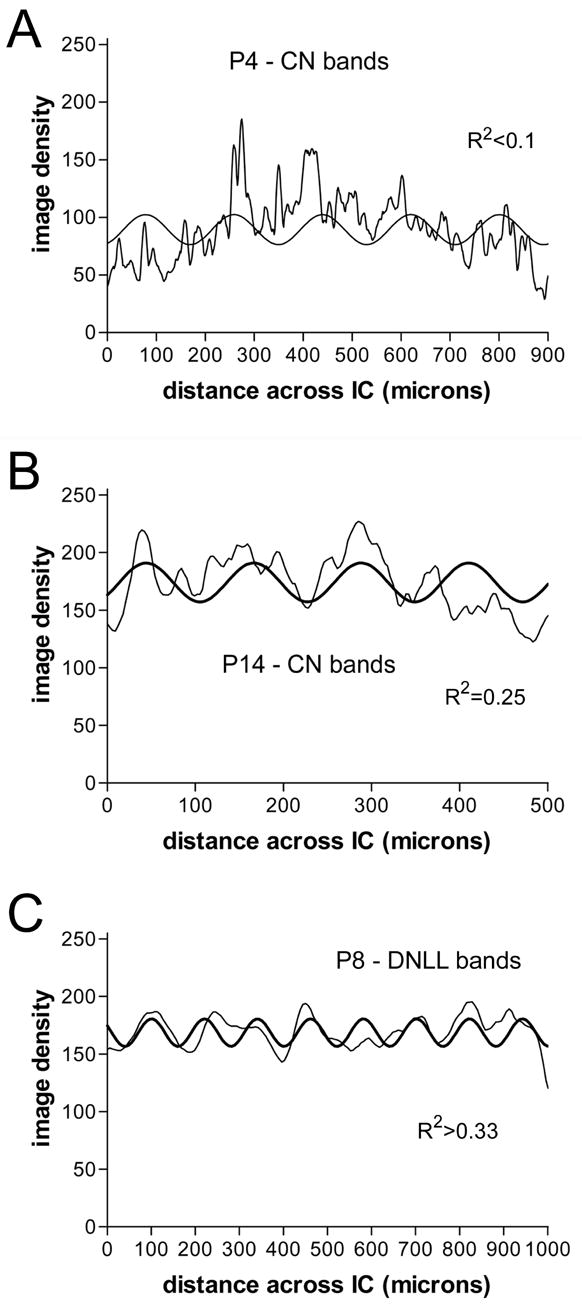
Line graphs of image density (thin graph line) orthogonal to plane of axonal layers with best fit sine curves (thick graph line) comparing pattern of developing afferent fibers for P4 and P14 cochlear nucleus (CN) cases (A and B, respectively) and a P8 DNLL case (C).
DiI-pins also were placed in the dorsal tegmental commissure (of Probst) to label crossed DNLL projections to the IC (Figure 6). Labeled fibers in these cases were relatively symmetric in the IC on each side and cells were retrogradely labeled in DNLL bilaterally (not shown). In P0 and P4 cases, labeled DNLL axons were profuse and distributed uniformly in the IC (Figure 6A), but the orientation of individual fibers was parallel to the fibrodendritic layers in the IC. By P7/8, labeling was concentrated in dense bands separated by intervening spaces with sparse labeling (Figure 6B). Nonlinear regression in the P0 case showed poor fit of labeling density to a sine curve (R2<0.1), whereas in the P7/8 case a good fit (Figure 5C, R2 ≥0.25) indicated marked progress in compartmentalization of the DNLL afferent bands as in the LSO cases.
Figure 6.
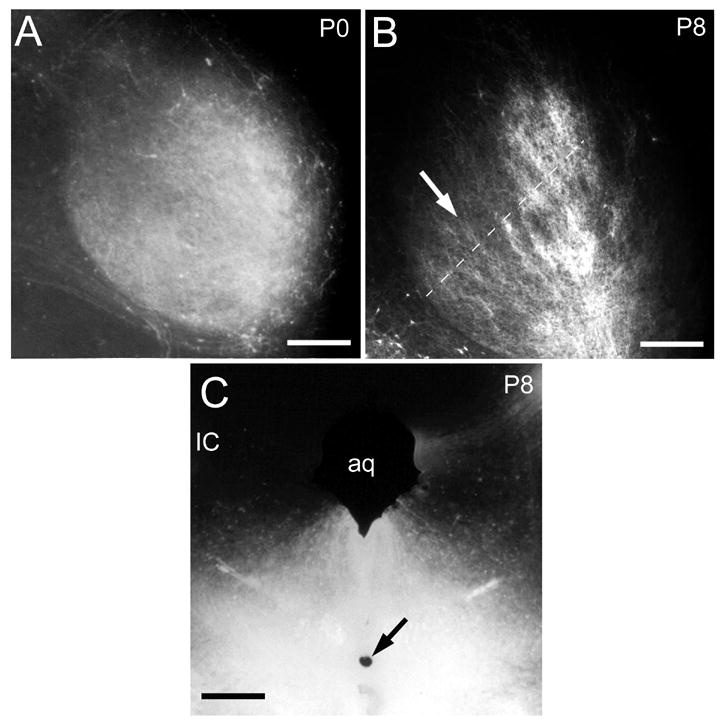
Epifluorescent images showing the pattern of afferent fibers in IC in a P0 (A) and P8 (B) case with a DiI-pin (inset, arrow) that labeled DNLL fibers at the dorsal tegmental commissure of Probst. Compare the diffusely distributed labeling in the IC at P0 with the banded organization at P8. Dashed line in B indicates orientation of selected field across IC labeling for image density analysis in Figure 5C. Calibration bars equal 200 μm. Other abbreviation: aq, cerebral aqueduct.
DISCUSSION
Axonal tracing with DiI was used to study postnatal development of banded afferent compartments within IC layers from three different hindbrain auditory nuclei in ferrets: LSO, DCN, and DNLL. The results show that LSO projections at P4 in ferrets are distributed diffusely within IC layers, but beginning by P7, and clearly by P14, they are segregated into a series of dense bands separated by parallel, but less densely labeled regions. Curve fitting of line density profiles across these afferent bands to a model sine wave confirmed a regular pattern of banded compartments with 90–110 μm center-to-center spacing. Similar analyses of P7 and P14 cases for DCN projections and of P7/8 cases for DNLL projections indicate that both of these projections also are beginning to segregate into banded compartments in the central nucleus of the IC by the end of the first postnatal week. Thus, the segregation of fibers from LSO, DCN, and DNLL into bands of afferent projections with the IC occurs well before hearing onset in ferrets. Any temporal differences in the maturation of banded compartments in IC layers must be shorter than the weekly intervals sampled.
Relation of afferent bands to IC laminar organization
The present findings indicate that assembly and refinement of banded afferent patterns for three functionally diverse projections to IC are achieved during the same postnatal time period. A previous ultrastructural study of synaptogenesis in ferret IC revealed a gradual progression in number of synapses and in their maturation that is consistent with such an overlapping temporal course of development of IC inputs (Brunso-Bechtold et al., 2006). LSO projections code binaural level differences with an inhibitory (glycinergic) ipsilateral projection and an excitatory contralateral projection to IC. DCN projections convey monaural information and are excitatory in IC. Contralateral DNLL projections are inhibitory (GABAergic) and may convey either binaural or monaural information to the IC (for review see Oliver and Shneiderman, 1989). The banded projection patterns of IC inputs define an organization of alternating layers receiving and integrating different information. Presumably neonatal development of this basic circuitry underlies the earliest monaural and binaural response properties of IC cells that can be recorded (Aitkin and Moore, 1975; Moore and Irvine, 1980, 1981; Ehret and Romand, 1992).
In this paper only the development of the banded pattern of IC afferents is addressed. Certainly development of other features of layered circuits such as axonal branching patterns, dendritic morphology, and synaptic arrangements may follow different time courses (see Cant, 1997 for review). Besides banded afferent compartments, there are other patterns of organization of afferent projections within the IC (e.g., Roth et al., 1978; Brunso-Bechtold et al., 1981; Cant and Benson, 2006). How development of other circuit features of IC layers is coordinated with the development of afferent bands remains to be determined.
Early time course of development of afferent circuits in IC
Ontogeny of brainstem and IC neurons has been described previously in rat (Altman and Bayer, 1980; 1981). Tritiated thymidine labeling for sources of afferent projections to IC marks a period from embryonic days 12–18 during which most of these cells are born. Kandler and Friauf (1993) showed in rat embryos that just a few days after the birth of cochlear nucleus cells their axonal projections reach the IC. Similarly, Gabriele et al. (2000a) showed that DNLL axons reach IC by embryonic day 22 (birth) in rat. Kudo et al. (1996) showed differential times of birth of LSO cells that project to the ipsilateral and contralateral IC in rat. Axons may wait for a synchronizing signal and then grow into IC layers simultaneously or some systems may grow in first and establish the initial laminar organization after which others follow, but these details have not been compared for different systems.
Early development of banded LSO projections in IC has been demonstrated previously with DiI-tracer in newborn kittens (Henkel et al., 2005). As in ferret, bands of LSO fibers in the kitten IC have segregated well before hearing onset. Similar afferent bands also were demonstrated by calbindin-immunostaining in IC in pre-hearing kitten. The general thickness and spacing of both DiI-labeled LSO projections and calbindin bands increased with postnatal growth of the IC, but remained the same relative size at all ages sampled. Because ferrets are more altricial than cats (average gestation of 42 days for ferrets compared to 65 days for cats), in the present study we were able to demonstrate the postnatal time course for refinement from an initially diffuse LSO projection to a pattern of afferent bands in IC layers.
Kandler and Friauf (1993) showed that cochlear nucleus axons arrive in the IC about one week prior to birth in rat pups after which time there is a progressive increase in the number of axons, their branching, and their topographic organization in the IC. The developmental time course of afferent band formation in IC was not described in that study. In adult rat (Malmierca et al., 2005) and cat (Oliver et al., 1997), DCN projections are segregated into banded compartments in the IC similar to those described for ipsilateral and contralateral projections from the LSO (Shneiderman and Henkel, 1987). The present results indicate that proliferating DCN axons begin to form bands in ferret IC in the second postnatal week concomitant to the formation of those from LSO.
Postnatal development of afferent bands was first described for crossed projections from the DNLL to rat IC (Gabriele et al., 2000a). DNLL axons first proliferate in rat IC and then become segregated into bands parallel to fibrodendritic layers between P4 and P8, about a week before hearing onset (P12). In ferrets, hearing onset begins at the end of the first postnatal month (Moore, 1982). In keeping with a more protracted postnatal developmental course, DNLL afferent projections to ferret IC, like those from LSO and DCN, form at least two weeks before hearing onset.
Thus, in a variety of circuits it has been demonstrated that auditory experience does not appear to be necessary for the formation of banded afferent compartments in IC. Indeed, other studies have demonstrated complex inhibitory and excitatory interactions after lemniscal and commissural stimulation in IC before hearing onset in the neonatal gerbil midbrain (Moore et al., 1998). A parallel course for the functional development of excitatory and inhibitory responses also has been demonstrated in neonatal gerbil LSO prior to auditory experience (Sanes, 1993).
Relation of development of afferent bands in IC to LSO development
Previously, we demonstrated that cells projecting to either the ipsilateral or contralateral IC are segregated more strictly along a shell to core gradient of LSO at birth than in adult ferrets (Henkel and Brunso-Bechtold, 1993). Phenotypic cell markers such as calcium-binding proteins and GABA (Henkel and Brunso-Bechtold, 1998; see also review by Sanes and Friauf, 2000) are distributed differentially along this same gradient in LSO but undergo a gradual transition in the second postnatal week after which their identity or segregation is less distinct. Thus, development of afferent bands in IC appears to be temporally congruent with neurochemical and topographic changes at the level of LSO.
Relation of pattern formation in IC layers to activity-dependent and activity-independent mechanisms
Critical periods for maturation of neural circuits are marked by particular events in sensory development. Obviously onset of sensory experience is one of those. Competition of inputs from each eye in the visual pathway to form eye-specific circuits is well described (Sretavan and Shatz, 1986; for recent review see Bence and Levelt, 2005). However, formation of ocular dominance columns in visual pathways takes place prior to sensory experience (Shatz, 1996; Katz and Crowley, 2002), as does formation of afferent bands in IC. In the visual pathway, waves of spontaneous activity across the retina regulate a variety of cellular and subcellular events that may contribute to early formation of ocular dominance columns (for general review see Sur and Rubenstein, 2005. Even so, spatial patterning of a variety of molecular guidance cues (e.g., chemoattractants and repellants such as the Eph-Ephrin family) may be independent of spontaneous activity (Crowley and Katz, 2002).
Spontaneous bursting and the role of activity-dependent and activity-independent mechanisms for circuit formation in the lower auditory brainstem have been a focus of studies of auditory development (for review see Friauf and Lohmann, 1999). Unilateral cochlear ablation in neonatal rats disrupts formation of DNLL afferent bands in IC before hearing onset (Gabriele et al., 2000b; Franklin et al., 2006) suggesting at least some role for spontaneous activity in the development of banded IC compartments. One possibility is that different afferent projections with complementary banded compartments in IC layers also compete for synaptic space and that this synaptic competition is dependent on spontaneous activity. In that case, formation and refinement of interdigitating banded layers might operate along Hebb rules and resemble formation of ocular dominance columns. Alternatively, spontaneous activity may be required for axons to respond to spatially distributed molecular markers. However, Leake et al. (2002) showed that sculpting of the projection pattern of primary afferent fibers in the cochlear nucleus begins prior to either spontaneous or evoked activity of the auditory nerve.
Whatever molecular and cellular signals are involved in band formation, whether or not they are dependent on spontaneous activity, the overlapping course of development of afferent bands from multiple sources in IC layers suggests that one input may not pioneer a template that guides subsequent development of other afferent inputs. It is possible that different afferent projections arrive at different times in the IC, but wait for other inputs before segregating into bands. Other features of layered circuits such as axonal branching patterns, dendritic morphology, and synaptic arrangements may be correlated with development of afferent bands or follow a different time course (see Cant, 1997 for review).
Conclusions
Axonal projections from LSO, DCN, and DNLL segregate into banded compartments along fibrodendritic layers in the IC before hearing begins and the time course for initial refinement of afferent bands from different sources overlaps during the second postnatal week in ferret. The concomitant development of banded patterns of axonal projections underscores the complexity of the competitive interactions that take place during early circuit formation in IC. Future experiments will begin to define the molecular and cellular signals mediating activity-dependent regulation of development of synaptic compartments in the IC layers.
Acknowledgments
Supported in part by NIH grant DC004412 and the Mr. and Mrs. A. Tab Williams, Jr. and Family Neuroscience Research and Program Development Endowment.
The authors would like to thank Stephanie Evans for her technical support and Stacy Wentworth who participated in collecting data from some of the earliest ages for this study.
ABBREVIATIONS
- aq
cerebral aqueduct
- das
dorsal acoustic stria
- CN
cochlear nucleus
- DCN
dorsal cochlear nucleus
- DiI
1,1'-dioctadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate
- DNLL
dorsal nucleus of the lateral lemniscus
- IC
inferior colliculus
- ICc
contralateral inferior colliculus
- ICi
ipsilateral inferior colliculus
- LSO
lateral superior olivary nucleus
- MSO
medial superior olivary nucleus
- P
postnatal day
- PVCN
posteroventral cochlear nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.Aitkin LM, Moore DR. Inferior colliculus. II. Development of tuning characteristics and tonotopic organization in central nucleus of the neonatal cat. J Neurophysiol. 1975;38:1208–1216. doi: 10.1152/jn.1975.38.5.1208. [DOI] [PubMed] [Google Scholar]
- 2.Altman JA, Bayer SA. Development of the brain stem in the rat. III Thymidine-radiographic study of the time of origin of neurons of the vestibular and auditory nuclei of the upper medulla. J Comp Neurol. 1980;194:877–904. doi: 10.1002/cne.901940410. [DOI] [PubMed] [Google Scholar]
- 3.Altman JA, Bayer SA. Time of origin of neurons of the rat inferior colliculus and the relations between cytogenesis and tonotopic order in the auditory pathway. Exp Brain Res. 1981;42:411–423. doi: 10.1007/BF00237506. [DOI] [PubMed] [Google Scholar]
- 4.Bence M, Levelt CN. Structural plasticity in the developing visual system. Prog Brain Res. 2005;147:125–139. doi: 10.1016/S0079-6123(04)47010-1. [DOI] [PubMed] [Google Scholar]
- 5.Brückner S, Rübsamen R. Binaural response characteristics in isofrequency sheets of the gerbil inferior colliculus. Hear Res. 1995;86:1–14. doi: 10.1016/0378-5955(95)00048-9. [DOI] [PubMed] [Google Scholar]
- 6.Brunso-Bechtold JK, Evans SD, Henkel CK. Synaptogenesis in the inferior colliculus of the pre-hearing postnatal ferret. Hear Res. 2006;218:1–4. doi: 10.1016/j.heares.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Brunso-Bechtold JK, Henkel CK. Development of auditory afferents to the central nucleus of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer-Verlag; 2005. pp. 537–558. [Google Scholar]
- 8.Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comparative Neurology. 1981;197:705–722. doi: 10.1002/cne.901970410. [DOI] [PubMed] [Google Scholar]
- 9.Cant NB. Structural development of the mammalian central auditory pathways. In: Rubel EW, Popper AN, Fay RR, editors. Development of the auditory system. New York: Springer-Verlag; 1997. pp. 315–413. [Google Scholar]
- 10.Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): Differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol. 2006;495:511–528. doi: 10.1002/cne.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowley JC, Katz LC. Ocular dominance development revisited. Curr Opin Neurobiol. 2002;12:104–109. doi: 10.1016/s0959-4388(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 12.Ehret G, Romand R. Development of tone response thresholds, latencies and tuning in the mouse inferior colliculus. Brain Res Dev Brain Res. 1992;67:317–326. doi: 10.1016/0165-3806(92)90233-m. [DOI] [PubMed] [Google Scholar]
- 13.Franklin SR, Brunso-Bechtold JK, Henkel CK. Unilateral cochlear ablation before hearing onset disrupts the maintenance of dorsal nucleus of the lateral lemniscus projection patterns in the rat inferior colliculus. Neurosci. 2006;143:105–115. doi: 10.1016/j.neuroscience.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitzpatrick KA. Cellular architecture and topographic organization of the inferior colliculus of the squirrel monkey. J Comp Neurol. 1975;164:185–208. doi: 10.1002/cne.901640204. [DOI] [PubMed] [Google Scholar]
- 15.Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297:187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- 16.Gabriele ML, Brunso-Bechtold JK, Henkel CK. Development of afferent patterns in the inferior colliculus of the rat: projection from the dorsal nucleus of the lateral lemniscus. J Comp Neurol. 2000a;416:368–382. doi: 10.1002/(sici)1096-9861(20000117)416:3<368::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 17.Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J Neurosci. 2000b;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamano K, Takeya T, Iwasaki N, Nakayama J, Ohto T, Okada Y. A quantitative study of the progress of myelination in the rat central nervous system, using the immunohistochemical method for proteolipid protein. Brain Res Dev Brain Res. 1998;108:287–293. doi: 10.1016/s0165-3806(98)00063-7. [DOI] [PubMed] [Google Scholar]
- 19.Henkel CK, Brunso-Bechtold JK. Laterality of superior olive projections to the inferior colliculus in adult and developing ferret. J Comparative Neurology. 1993;331:458–468. doi: 10.1002/cne.903310403. [DOI] [PubMed] [Google Scholar]
- 20.Henkel CK, Brunso-Bechtold JK. Calcium-binding proteins and GABA reveal spatial segregation of cell types within the developing lateral superior olivary nucleus of the ferret. Microsc Res Tech. 1998;41:234–245. doi: 10.1002/(SICI)1097-0029(19980501)41:3<234::AID-JEMT7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 21.Henkel CK, Fuentes-Santamaria V, Alvarado JC, Brunso-Bechtold JK. Quantitative measurement of afferent layers in the ferret inferior colliculus: DNLL projections to sublayers. Hear Res. 2003;177:32–42. doi: 10.1016/s0378-5955(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 22.Henkel CK, Gabriele ML, McHaffie JG. Quantitative assessment of developing afferent patterns in the cat inferior colliculus revealed with calbindin immunohistochemistry and tract tracing methods. Neuroscience. 2005;136:945–955. doi: 10.1016/j.neuroscience.2005.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewett DL, Romano MN. Neonatal development of auditory system potentials averaged from the scalp of rat and cat. Brain Res. 1972;36:101–115. doi: 10.1016/0006-8993(72)90769-x. [DOI] [PubMed] [Google Scholar]
- 24.Kandler K, Friauf E. Pre- and postnatal development of efferent connections of the cochlear nucleus in the rat. J Comp Neurol. 1993;328:161–184. doi: 10.1002/cne.903280202. [DOI] [PubMed] [Google Scholar]
- 25.Katz LC, Crowley JC. Development of cortical circuits: lessons from ocular dominance columns. Nat Rev Neurosci. 2002;3:34–42. doi: 10.1038/nrn703. [DOI] [PubMed] [Google Scholar]
- 26.Keiger JC, Henkel CK, Brunso-Bechtold JK. Assoc Res Otolaryngol Abs. Vol. 26. 2005. Normal postnatal development of banded afferent projections to the inferior colliculus of the ferret from the superior olivary complex; p. 844. [Google Scholar]
- 27.Kelly JB, Liscum A, Van Adel B, Ito M. Projections from the superior olive and lateral lemniscus to tonotopic regions of the rat's inferior colliculus. Hear Res. 1998;116:43–54. doi: 10.1016/s0378-5955(97)00195-0. [DOI] [PubMed] [Google Scholar]
- 28.Kudo M, Kitao Y, Okoyama S, Moriya M, Kawano J. Crossed projection neurons are generated prior to uncrossed projection neurons in the lateral superior olive of the rat. Dev Brain Res. 1996;95:72–78. doi: 10.1016/0165-3806(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 29.Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Looney GA, Elberger AJ. Myelination of the corpus callosum in the cat: time course, topography, and functional implications. J Comp Neurol. 1986;248:336–347. doi: 10.1002/cne.902480304. [DOI] [PubMed] [Google Scholar]
- 31.Lukas JR, Aigner M, Denk M, Heinzl H, Burian M, Mayr R. Carbocyanine postmortem neuronal tracing. Influence of different parameters on tracing distance and combination with immunocytochemistry. J Histochem Cytochem. 1998;46:901–910. doi: 10.1177/002215549804600805. [DOI] [PubMed] [Google Scholar]
- 32.Malmierca MS, Saint Marie RL, Merchan MA, Oliver DL. Laminar inputs from dorsal cochlear nucleus and ventral cochlear nucleus to the central nucleus of the inferior colliculus: two patterns of convergence. Neuroscience. 2005;136:883–894. doi: 10.1016/j.neuroscience.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 33.Merzenich MM, Reid MD. Representation of the cochlea within the inferior colliculus of the cat. Brain Res. 1974;77:397–415. doi: 10.1016/0006-8993(74)90630-1. [DOI] [PubMed] [Google Scholar]
- 34.Moore DR, Irvine DR. Development of binaural input, response patterns, and discharge rate in single units of the cat inferior colliculus. Exp Brain Res. 1980;38:103–108. doi: 10.1007/BF00237936. [DOI] [PubMed] [Google Scholar]
- 35.Moore DR, Irvine DR. Development of responses to acoustic interaural intensity differences in the cat inferior colliculus. Exp Brain Res. 1981;41:301–309. doi: 10.1007/BF00238887. [DOI] [PubMed] [Google Scholar]
- 36.Moore DR. Late onset of hearing in the ferret. Brain Res. 1982;253:309–311. doi: 10.1016/0006-8993(82)90698-9. [DOI] [PubMed] [Google Scholar]
- 37.Moore DR, Kotak VC, Sanes DH. Commissural and lemniscal synaptic input to the gerbil inferior colliculus. J Neurophysiol. 1998;80:2229–2236. doi: 10.1152/jn.1998.80.5.2229. [DOI] [PubMed] [Google Scholar]
- 38.Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol. 1997;382:215–229. doi: 10.1002/(sici)1096-9861(19970602)382:2<215::aid-cne6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 39.Oliver DL, Morest DK. The central nucleus of the inferior colliculus in the cat. J Comp Neurol. 1984;222:237–264. doi: 10.1002/cne.902220207. [DOI] [PubMed] [Google Scholar]
- 40.Oliver DL, Shneiderman A. The anatomy of the inferior colliculus: a cellular basis for integration of monaural and binaural information. In: Altschuler RA, editor. Neurobiology of hearing: The central auditory system. II 1989. [Google Scholar]
- 41.Rockel AJ, Jones EG. The neuronal organization of the inferior colliculus of the adult cat. I: The central nucleus. J Comp Neurol. 1973;147:11–60. doi: 10.1002/cne.901470103. [DOI] [PubMed] [Google Scholar]
- 42.Roth GL, Aitkin LM, Andersen RA, Merzenich MM. Some features of the spatial organization of the central nucleus of the inferior colliculus of the cat. J Comp Neurol. 1978;182:661–680. doi: 10.1002/cne.901820407. [DOI] [PubMed] [Google Scholar]
- 43.Sanes DH. The development of synaptic function and integration in the central auditory system. J Neurosci. 1993;13:2627–2637. doi: 10.1523/JNEUROSCI.13-06-02627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanes DH, Friauf E. Development and influence of inhibition in the lateral superior olivary nucleus. Hear Res. 2000;147:46–58. doi: 10.1016/s0378-5955(00)00119-2. [DOI] [PubMed] [Google Scholar]
- 45.Semple MN, Aitkin LM. Representation of sound frequency and laterality by units in central nucleus of cat inferior colliculus. J Neurophysiol. 1979;42:1626–1639. doi: 10.1152/jn.1979.42.6.1626. [DOI] [PubMed] [Google Scholar]
- 46.Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol. 1987;266:519–534. doi: 10.1002/cne.902660406. [DOI] [PubMed] [Google Scholar]
- 47.Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system. J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- 48.Sparks DL, Lue LF, Martin TA, Rogers J. Neural tract tracing using Di-I: a review and a new method to make fast Di-I faster in human brain. J Neurosci Methods. 2000;103:3–10. doi: 10.1016/s0165-0270(00)00291-0. [DOI] [PubMed] [Google Scholar]
- 49.Sretavan DW, Shatz CJ. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. J Neurosci. 1986;6:234–251. doi: 10.1523/JNEUROSCI.06-01-00234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- 51.Wenstrup JJ, Ross LS, Pollak GD. Binaural response organization within a frequency-band representation of the inferior colliculus: implications for sound localization. J Neurosci. 1986;6:962–973. doi: 10.1523/JNEUROSCI.06-04-00962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


