Abstract
We performed this study to determine the predictors of early and long-term survival in the surgical treatment of tuberculous pericarditis and to examine the risks of pericardiectomy and the functional outcome in patients after surgery.
A retrospective analysis was undertaken in 36 consecutive patients, 26 female and 10 male, with a mean age 32.2 ± 16.3, who underwent pericardiectomy for chronic constrictive pericarditis from February 1985 to February 2002. All patients received antitubercular therapy in the postoperative period.
The operative mortality rate was 6% (2 patients); the cause of death in both cases was severe low-cardiac-output syndrome. Nonfatal intraoperative complications affected 3 patients (8%). The median stay in the intensive care unit was 3.7 ± 3.1 days. The median hospital stay was 14 ± 2.6 days. The median ventilation time was 11.9 ± 1.8 hours. The median volume of blood transfused was 2.1 ± 1.6 units.
Advanced age, atrial fibrillation, concomitant tricuspid insufficiency, inotropic support, and low cardiac output were significant negative predictors of survival, according to univariate analysis. There were 4 late deaths. Actuarial survival at 5 years was 75.9% ± 9.14%. At the 1-year follow-up examination, improved functional status was noted in 88% of patients.
We suggest that pericardiectomy be performed early and as radically as possible, in an effort to prevent chronic illness. A combination of chemotherapy and surgery yields gratifying results in the treatment of tuberculous pericarditis. (Tex Heart Inst J 2003;30:180–5)
Key words: Pericardiectomy; pericarditis, constrictive/surgery; treatment outcome; tuberculosis, cardiovascular
Tuberculous pericarditis (TBP), a rare but important complication of infection with Mycobacterium tuberculosis, can progress rapidly to constriction. 1 Constrictive calcific pericarditis, although still relatively uncommon, 2 has once again become a real clinical entity—due in part to the emergence of drug-resistant strains of tuberculosis (TBC) in association with acquired immunodeficiency syndrome. 3
In a retrospective study of 36 patients who had undergone pericardiectomy for constrictive tuberculous pericarditis, we analyzed patients' clinical features, together with predictors of their early and late-term survival. We discuss the current status of therapy in the light of modern cardiovascular surgery, and we review recent experience with tuberculous pericarditis as presented in the literature.
Patients and Methods
The study group consisted of 36 consecutive patients with constrictive tuberculous pericarditis who underwent surgery from February 1985 to February 2002 at Kosuyolu Heart and Research Hospital.
There were 26 female (72%) and 10 male (28%) patients with a mean age of 32.2 ± 16.3 years (range, 15 to 72 years). Their presenting signs and symptoms are summarized in Table I. The clinical spectrum of the patients was affected by the duration of their pericardial constriction. The mean constrictive period, after clinically proven TBC, was 2.3 ± 1.3 years (range, 0.5 to 6 years). Constrictive TBP led to severe heart failure in 21 (58%) patients, and rapid deterioration in clinical status was characteristic of that group.
TABLE I. Presenting Symptoms of 36 Patients with Constrictive Tuberculous Pericarditis
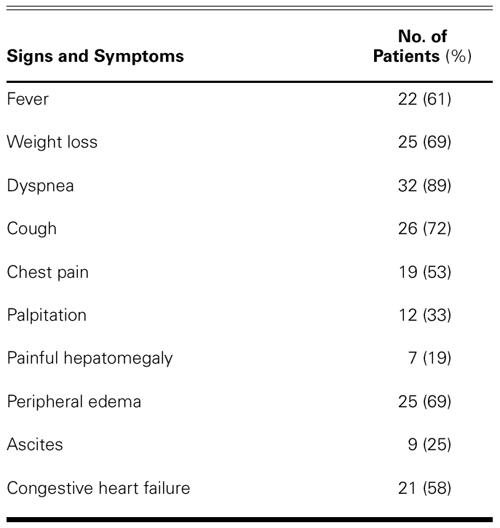
Preoperatively, there were no patients in New York Heart Association (NYHA) functional class I or II, 21 patients (58%) in class III, and 15 (42%) in class IV (Fig. 1).
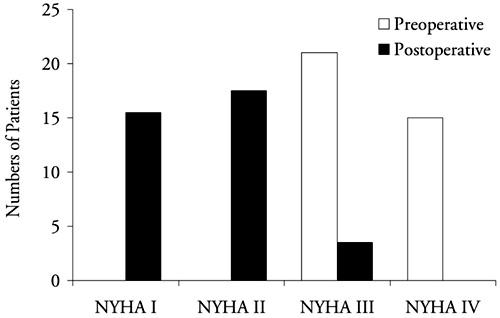
Fig. 1 Preoperative and early postoperative New York Heart Association (NYHA) functional status of 36 patients.
On electrocardiography, low voltage was detected in 13 patients (36%) and atrial fibrillation was found in 25 (69%). Chest radiography showed pericardial calcification in 16 patients (44%) and pleural effusion in 22 (61%). The mean cardiothoracic ratio was 0.54 ± 0.2. Four patients had macroscopic foci of TBC in the pericardium or nearby, and 17 patients had evidence of active or treated TBC that affected the lungs or the pericardium, or both. All but 2 of the 36 patients were PPD (purified protein derivative of tuberculin) positive, and those 2 were anergic. Myocarditis and pericarditis with tamponade were associated with disseminated TBC. All patients had received antitubercular (anti-TBC) therapy before the present admission.
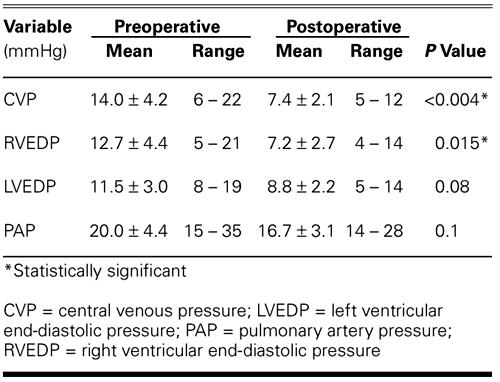
Pericardial effusion revealed by echocardiography was moderate in 7 patients and severe in 2. The mean ejection fraction for the group of 36 was 0.62 ± 0.8 (range, 0.30 to 0.70), as determined by echocardiography. Thirty patients (83%) underwent catheterization. The patients' hemodynamic status is summarized in Table II.
TABLE II. Comparison of Preoperative and Postoperative Hemodynamic Results in 30 Patients
In all cases, pericardiectomy was performed through a median sternotomy. Total pericardiectomy was defined as wide excision of the pericardium from all surfaces of the heart and major intrapericardial vessels, with the phrenic nerves defining the posterior extent of pericardial resection. Constricting layers of epicardium were removed if possible. The dissection was started at the ascending aorta, the lateral and posterior walls of the left ventricle and the pulmonary veins, and the pulmonary artery; then it was continued over the diaphragmatic surface of the pericardium. Finally, the pericardium was resected over the free wall of the right ventricle, and then over the right atrium and venae cavae.
The mean operative time was 104.6 ± 30.1 min (range, 65 to 195 min). Total pericardiectomy was carried out in 33 patients (92%). In 3 patients (8%), dense myoepicardial adhesions and calcification limited the procedure to partial pericardiectomy. Cardiopulmonary bypass (CPB) was used in 3 patients (8%). Concomitant operations (atrial septal defect closure and coronary artery bypass grafting) were undertaken in 2 patients to repair congenital or acquired heart disease.
Postoperative diagnosis was made on the basis of tubercle bacilli in pericardial fluid or in a histologic section of the pericardium, or on proof of TBC elsewhere in a patient with otherwise unexplained pericarditis. Postoperatively, medical treatment consisted of triple-drug therapy for at least 9 months (isoniazid, rifampin, and ethambutol). During the first 2 months, some patients received streptomycin as a substitute for ethambutol; at the conclusion of this period, ethambutol was given for the remaining 7 months. All patients received pyrazinamide during the first 2 postoperative months.
Data were analyzed using SPSS version 10.0 (SPSS, Inc.; Chicago, Ill). Regression analysis was performed using 45 clinical variables. Data are presented as the mean ± standard deviation. A P value <0.05 was considered significant for all statistical calculations. Forty-five clinical variables were analyzed by use of the Cox proportional hazards method, to determine negative predictors of long-term survival (Table III). Actuarial survival was calculated by the Kaplan-Meier method.
TABLE III. Preoperative, Postoperative, and Follow-Up Variables

Results
The 30-day operative mortality rate was 6% (2 patients); the cause of death in both cases was severe low-cardiac-output syndrome.
Nonfatal intraoperative complications affected 3 patients (8%): intraoperative bleeding occurred in 2 patients due to laceration of the right atrium and in 1 due to laceration of the right ventricle. Another patient was difficult to wean from CPB, so an intraaortic balloon pump was used. Catecholamines were used in 31 patients (86%), and low-dose dopamine was used for prophylaxis against low-output syndrome in all patients.
The median ventilation time was 11.9 ± 4.8 hours. The median volume of blood transfused was 2.1 ± 1.6 units. The median stay in the intensive care unit was 3.7 ± 3.1 days. The median hospital stay was 14 ± 2.6 days.
Postoperative improvements in hemodynamic results are shown in Table II. Of these, the improvements in mean central venous pressure (from 14.0 ± 4.2 to 7.4 ± 2.1 mmHg; P <0.004) and in mean right ventricular end-diastolic pressure (from 12.7 ± 4.4 to 7.2 ± 2.7 mmHg; P = 0.015) are statistically significant.
Advanced age (P = 0.019), atrial fibrillation (P = 0.028), concomitant tricuspid insufficiency (P = 0.046), inotropic support (P = 0.006), and low cardiac output (P = 0.012) were significant negative predictors of survival, according to univariate analysis. Four patients were lost to follow-up, and the remaining 30 underwent 132.6 patient-years of follow-up. The median follow-up time was 47.3 ± 26.8 months. At the 1-year follow-up, improved functional status was noted in 88% of the surviving patients, and 31 of the 34 surviving patients (91%) were in NYHA class I or II (Fig. 1). In the late postoperative period, no patient died of a cardiac cause. The late mortality rate was 11.8%: 4 of the surviving 34 patients died from complications of disseminated TBC. The actuarial survival rate in all 36 patients at 5 years was 75.9% ± 9.14% (Fig. 2).
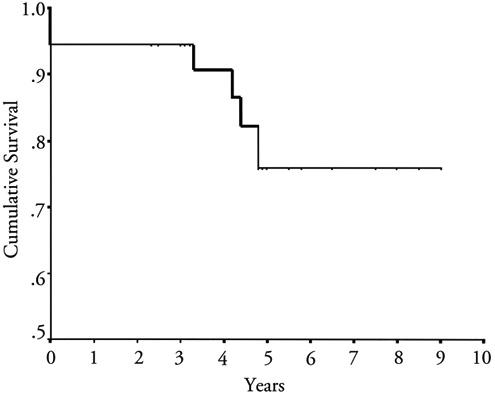
Fig. 2 Actuarial survival for all patients who underwent pericardiectomy (including the 2 who died perioperatively).
Discussion
Constrictive pericarditis is an uncommon disorder with various causes. 4,5 Chronic constrictive pericarditis is an inflammatory process that involves both fibrous and serous layers of the pericardium, leading to pericardial thickening and constriction of the ventricles. 6
Myocarditis and pericarditis are life-threatening complications of disseminated tuberculous disease. In industrialized countries such as the United States, constrictive calcified pericarditis in association with treated pulmonary TBC is rare, 1 due to the decreased incidence of tuberculosis in modern cardiovascular practice. 4 Yet TBC remains a leading cause of pericarditis in some nonindustrialized countries, 2,3 such as those of sub-Saharan Africa, where infection with human immunodeficiency virus (HIV) is pandemic. Symptomatic pericardial involvement is frequently associated with stage C of HIV infection. 3 Even in the United States, the association of TBP with HIV will certainly lead to a greater number of TBC cases in the near future.
Tuberculous pericarditis affects 1% to 2% of all patients with TBC by direct extension from the mediastinal lymph nodes and, occasionally, by hematogenous spread or by contiguous spread from the myocardium. 2 It nearly always occurs in association with another focus of TBC, but that other focus is sometimes silent. Tuberculosis is responsible for approximately 4% of cases of acute pericarditis, 7% of cases of cardiac tamponade, and, in older studies, 6% of instances of constrictive pericarditis. 6
Certain forces determine whether or not fibrinous pericarditis develops into adhesive pericarditis and how rapidly constriction progresses. Interestingly, pericardial mesothelial cells harbor a plasminogen inhibitor and produce adenosine, but do not express tissue factor to activate the extrinsic coagulation pathway. Tuberculosis causes a fibrinous suppurative exudate that eventually produces adhesion between the visceral and parietal layers and thickened, calcific constrictive pericarditis.
Most patients who have TBC myocarditis present with heart failure, and constrictive pericarditis causes severe heart failure. 7–9 The encasement of the heart within a nonpliable pericardium results in characteristic pathophysiologic effects, including impaired diastolic filling of the ventricles, exaggerated ventricular interdependence, and dissociation of intracardiac and intrathoracic pressures during respiration. 8 Constrictive pericarditis typically presents with chronic, insidious signs and symptoms of predominantly systemic venous congestion.
In cases of constrictive pericarditis, magnetic resonance imaging (MRI) reveals thickened pericardium, and cardiac catheterization indicates diastolic equalization of pressures in the 4 chambers. The jugular venous pulse shows a dominant “Y” descent that coincides with early diastolic flow in the superior vena cava, and Doppler echocardiography can reveal mitral and tricuspid forward flow demonstrative of restriction. 10 The diagnosis of constrictive pericarditis remains a challenge, however, because the physical and hemodynamic findings mimic those of restrictive cardiomyopathy. Various diagnostic advances over the years have enabled us to differentiate between these 2 conditions. Most cases can be diagnosed by cardiac catheterization, transthoracic or transesophageal echocardiography, central venous (hepatic and pulmonary) and transvalvular Doppler measurements, and MRI, thereby eliminating the need for diagnostic thoracotomy. 5 Computed tomography and MRI can delineate the abnormal pericardial thickness found in constrictive pericarditis. The association of characteristic hemodynamic changes with pericardial thickness greater than 3 mm usually confirms the diagnosis. 10 Nuclear ventriculograms reveal rapid ventricular filling in constrictive pericarditis, which differentiates that disorder from restrictive cardiomyopathy. Endomyocardial biopsy helps further, by distinguishing between the various types of restrictive cardiomyopathy.
In patients with tuberculous pericarditis, the recommendation is that triple-drug antituberculous therapy be administered for a minimum of 9 months. 7,11 In patients in whom pericardial effusion persists or recurs despite the use of anti-TBC drugs, 3 months of corticosteroid therapy may be a useful adjunct. Although medical treatment may temporarily alleviate symptoms of heart failure, TBP patients do poorly without pericardiectomy. If there is poor resolution of effusion after several weeks of anti-TBC therapy, early pericardiectomy is recommended in an attempt to prevent the development of constriction. 7,11,12
Pericardiectomy is the only accepted curative treatment for improving cardiac hemodynamics in TBP. Care must be taken to resect every constrictive epicardial layer to avoid persistent pericardial constriction. The constricting envelope surrounds the entire heart and interferes with diastolic filling. The disease affects the filling of all cardiac chambers, and, as the constriction progresses, only early diastolic filling is possible. In patients with compromise of cardiac function, surgery to remove the constricting envelope is the only effective long-term treatment.
Median sternotomy provides good exposure of the right atrium and the venae cavae, and it enables extensive removal of the pericardium. 13–15 Previously, a left anterolateral thoracotomy was used, 13 but that approach renders difficult the institution of CPB in the event of extensive bleeding. Through the median sternotomy, the patient can easily be connected to CPB, without which extensive bleeding cannot be controlled. Cardiopulmonary bypass aids surgical dissection by emptying the ventricular cavities, which clearly defines the appropriate plane of dissection and facilitates the management of inadvertent cardiac injury. Although we have had experience in video-assisted thoracoscopic pericardiectomy in a series of cases of effusive pericarditis, we have not attempted video-assisted thoracoscopic pericardiectomy in TBP. We prefer conventional open pericardiectomy via the median sternotomy, which enables a safer, wider, and more effective approach. 16
Regardless of the surgical approach or use of CPB, investigators have reported normalization of cardiac hemodynamics after radical pericardiectomy. 12,13 Postoperatively, central venous pressure tends to decrease in 2 to 4 days and to normalize in 4 weeks. On late follow-up Doppler echocardiography, Senni and colleagues 9 found that left ventricular diastolic filling remained abnormal in 43% of patients who had undergone radical pericardiectomy. It has been suggested 9 that the abnormal ventricular compliance is due to myocardial alterations and that resection of the pericardium overlying the right atrium and the great veins might not be essential. However, Culliford and colleagues 17 theorized that delayed improvement and persistent symptoms of pericardial constriction are most commonly the results of incomplete decortication. The cause of persistent abnormal ventricular end-diastolic pressure in the early postoperative period is unclear; however, inadequate resection cannot be excluded. By studying left ventricular pressure-volume loops, Koruda and coworkers 8 found that total pericardiectomy normalized both the diastolic filling pattern and systolic function.
A small but not insignificant portion of patients with constrictive pericarditis will develop low-output syndrome after pericardiectomy, regardless of the operative approach or the extent of pericardial resection. 7 Despite extensive pericardiectomy, there have always been some early deaths due to low-output syndrome. This is because outcome is related not only to the extent of surgery but to myocardial involvement. Autopsy findings indicate that myocardial fibrosis and atrophy may result from chronic constrictive pericarditis. 7 Low-output syndrome can also be caused by changes in cardiac architecture: long periods of myocardial compression contribute to “remodeling” of the ventricles and to greater involvement of the myocardium in patients who have undergone long periods of symptomatic pericardial constriction. Worsening tricuspid regurgitation can be observed as a result of postoperative right ventricular dilatation. 18 Mitral insufficiency after pericardiectomy, observed by means of transesophageal echocardiography, was thought by Buckingham and colleagues 19 to be due to elongation of the papillary muscles. In most cases, a low-output state after pericardiectomy gradually improves during hospitalization. 14
Conclusion
In this study, advanced age, atrial fibrillation, concomitant tricuspid insufficiency, inotropic support, and low cardiac output were predictors of poor survival. We suggest that pericardiectomy be performed early and as radically as possible, in an effort to prevent chronic illness. A combination of chemotherapy and operation yields gratifying results in the treatment of tuberculous pericarditis.
Footnotes
Address for reprints: Dr. Nilgun Bozbuga, Kosuyolu Heart and Research Hospital, Department of Cardiovascular Surgery, Istanbul, Turkey
E-mail: nbozbuga@kosuyolu.gov.tr
References
- 1.Kirklin JW, Barrett-Boyes BG. Cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indications. Vol 2. 2nd ed. New York: Churchill Livingstone, Inc.; 1993. p. 1683–98.
- 2.Afzal A, Keohane M, Keeley E, Borzak S, Callender CW, Iannuzzi M. Myocarditis and pericarditis with tamponade associated with disseminated tuberculosis. Can J Cardiol 2000;16:519–21. [PubMed]
- 3.Hakim JG, Ternouth I, Mushangi E, Siziya S, Robertson V, Malin A. Double blind randomised placebo controlled trial of adjunctive prednisolone in the treatment of effusive tuberculous pericarditis in HIV seropositive patients. Heart 2000;84:183–8. [DOI] [PMC free article] [PubMed]
- 4.Ling LH, Oh JK, Schaff HV, Danielson GK, Mahoney DW, Seward JB, Tajik AJ. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999;100:1380–6. [DOI] [PubMed]
- 5.Myers RB, Spodick DH. Constrictive pericarditis: clinical and pathophysiologic characteristics. Am Heart J 1999;138 (2 Pt 1):219–32. [DOI] [PubMed]
- 6.Fowler NO. Tuberculous pericarditis. JAMA 1991;266:99–103. [PubMed]
- 7.DeValeria PA, Baumgartner WA, Casale AS, Greene PS, Cameron DE, Gardner TJ, et al. Current indications, risks, and outcome after pericardiectomy. Ann Thorac Surg 1991;52:219–24. [DOI] [PubMed]
- 8.Kuroda H, Sakaguchi M, Takano T, Tsunemoto H, Shino-hara M, Fukaya Y, Amano J. Intraoperative monitoring of pressure-volume loops of the left ventricle in pericardiectomy for constrictive pericarditis. Ann Thorac Surg 1996;112:198–9. [DOI] [PubMed]
- 9.Senni M, Redfield MM, Ling LH, Danielson GK, Tajik AJ, Oh JK. Left ventricular systolic and diastolic function after pericardiectomy in patients with constrictive pericarditis: Doppler echocardiographic findings and correlation with clinical status. J Am Coll Cardiol 1999;33:1182–8. [DOI] [PubMed]
- 10.Mehta A, Mehta M, Jain AC. Constrictive pericarditis. Clin Cardiol 1999;22:334–44. [DOI] [PMC free article] [PubMed]
- 11.Suwan PK, Potjalongsilp S. Predictors of constrictive pericarditis after tuberculous pericarditis. Br Heart J 1995;73:187–9. [DOI] [PMC free article] [PubMed]
- 12.Quale JM, Lipschik GY, Heurich AE. Management of tuberculous pericarditis. Ann Thorac Surg 1987;43:653–5. [DOI] [PubMed]
- 13.Astudillo R, Ivert T. Late results after pericardectomy for constrictive pericarditis via left thoracotomy. Scand J Thorac Cardiovasc Surg 1989;23:115–9. [DOI] [PubMed]
- 14.Omoto T, Minami K, Varvaras D, Bothig D, Korfer R. Radical pericardiectomy for chronic constrictive pericarditis. Asian Cardiovasc Thorac Ann 2001;9:286–90.
- 15.Tirilomis T, Unverdorben S, von der Emde J. Pericardectomy for chronic constrictive pericarditis: risks and outcome. Eur J Cardiothorac Surg 1994;8:487–92. [DOI] [PubMed]
- 16.Daglar B, Bozbuga N, Isik O, Akinci E, Guler M, Eren E, et al. Videothoracoscope assisted cardiothoracic surgery. Eur J Card Intervent (Cor Europeum) 1999;7:173–6.
- 17.Culliford AT, Lipton M, Spencer FC. Operation for chronic constrictive pericarditis: Do the surgical approach and degree of pericardial resection influence the outcome significantly? Ann Thorac Surg 1980;29:146–52. [DOI] [PubMed]
- 18.Johnson TL, Bauman WB, Josephson RA. Worsening tricuspid regurgitation following pericardiectomy for constrictive pericarditis. Chest 1993;104:79–81. [DOI] [PubMed]
- 19.Buckingham RE Jr, Furnary AP, Weaver MT, Floten HS, Davis RF. Mitral insufficiency after pericardiectomy for constrictive pericarditis. Ann Thorac Surg 1994;58:1171–4. [DOI] [PubMed]


