Abstract
We argue that many similar findings observed in cognitive, affective, and social neuroimaging research may compose larger processes central to generating self-relevance. In support of this, recent findings from these research domains were reviewed to identify common systemic activation patterns. Superimposition of these patterns revealed evidence for large-scale supramodal processes, which are argued to mediate appraisal of self-relevant content irrespective of specific stimulus types (e.g. words, pictures) and task domains (e.g. induction of reward, fear, pain, etc). Furthermore, we distinguish between two top-down sub-systems involved in appraisal of self-relevance, one that orients pre-attentive biasing information (e.g. anticipatory or mnemonic) to salient or explicitly self-relevant phenomena, and another that engages introspective processes (e.g. self-reflection, evaluation, recollection) either in conjunction with or independent of the former system. Based on aggregate patterns of activation derived from the reviewed studies, processes in a ventral MPFC—subcortical network appear to track with the former pathway, and processes in a dorsal MPFC—cortical—subcortical network with the latter. As a whole, the purpose of this framework is to re-conceive the functionality of these systems in terms of supramodal processes that more directly reflect the influences of relevance to the self.
Keywords: appraisal, self-relevance, cortical–subcortical systems, introspection
Introduction
What is and what is not self-relevant may vary widely from one human to another, yet several lines of recent neuroimaging research implicate the presence of dedicated brain systems central to appraising the self-relevant content of one’s environment and one’s conscious mental events. These systems are attuned to the detection of salient environmental phenomena that convey significance to an organism (e.g. unexpected movement), yet perhaps solely in humans, these systems are able to instantiate elaborate self-relevant information from past experience to create abstract associations with stimuli, or, to instantiate such information independent of external stimuli altogether. In this way, we consider salience simply as a bottom-up propagation of sensory information sufficient for allocation of attention, whereas self-relevance is defined as top-down and generated by two integrative sub-systems, one that orients pre-attentive biasing information (e.g. anticipatory or mnemonic) to salient or explicitly self-relevant phenomena, and another that engages introspective processes (e.g. self-reflection, evaluation, recollection) either in conjunction with or independent of the former pathway. Our objective in this review is to identify convergent neuroimaging evidence for these top-down sub-systems and put forth an integrative model of the neural substrata underlying appraisal of self-relevance (ASR).
We argue that many similar findings observed in affective, cognitive, and social neuroimaging (ACS) research may compose a larger overlapping pattern indicative of central brain systems attuned to ASR. First, the component processes mediated by these systems are conceptualized as functioning at a supramodal level (Northoff and Bermpohl 2004; Northoff, et al. 2006), where supramodal is defined generally as a class of neural processes that respond to information independent of sensory modality, and as demonstrated in many neuroimaging studies, independent of stimulus type (e.g. words, pictures) and perhaps most importantly of task domain (e.g. induction of reward or fear appraisal). Second, the collective supramodal processes outlined in this review appear primarily sensitive, or specialized, to generating self-relevance. Based on recent evidence from diverse ACS paradigms requiring appraisal of self-relevant information, these processes appear localized to brain regions within the overall system. The anterior medial prefrontal cortex (MPFC) in particular is responsive to self-relevant information, though similar response patterns have also recently been characterized across extensive cortical (posterior/anterior cingulate) and subcortical systems. Contrary to the frequent observation that brain regions within these systems appear selective to information of a relatively narrow range (e.g. a ventral MPFC—amygdalar ‘fear circuit’ (Lang, et al. 2000), the growing presence of highly overlapping MPFC—cortical—subcortical findings across seemingly disparate ACS research instead points to the supramodal processing capacity and primacy of ASR inherent to the functions of these regions.
The component processes underlying ASR evoke large-scale neural signatures that are difficult to differentiate by specific functional contribution, though recent reviews have endeavored to do so (Amodio and Frith 2006; Northoff and Bermpohl 2004; Northoff, et al. 2006; Ochsner, et al. 2005; Phillips, et al. 2003a; Phillips, et al. 2003b). This difficulty may arise from the two discrete yet rapidly integrative sources of input that continuously inform ASR: extero/interoceptive information (e.g. sensory, somatic, autonomic) and introspective information (e.g. thoughts, memories). However, collective findings from recent neuroimaging research, including our own, have begun to decompose these input streams in two ways.
First, the observation that many different task domains evoke common neural response patterns suggests that the underlying supramodal systems are sensitive to self-relevant features shared by these domains. What are these features? In ACS research, task domains that convey self-relevance fall into broad cognitive-affective categories inclusive of reward (e.g. financial gain), fear (e.g. conditioned response), pain (e.g. shock), as well as affective cues spanning the emotion spectrum (e.g. using faces, scenes, words). Taken together, these task domains are inherently self-relevant to humans, as by design they require feature-based appraisal of stimuli that are predisposed to bias an internal state of a particular emotional valence (e.g. reward, fear) or arousal level. In doing so, these paradigms orient the participant (e.g. through successive presentations of an aversive picture or anticipation of a painful stimulus) to a specific biasing feature of the stimulus, where collectively these features are considered self-relevant (see Table 1 for examples of tasks requiring feature-based appraisal). Studies examining these task domains consistently identify overlapping cortical—subcortical systems inclusive of the ventral medial prefrontal cortex (vMPFC) and anterior cingulate (ACC), dorsal—ventral striatum (Nacc), amygdala (Amg), and insula (Davidson and Irwin 1999; Phillips, et al. 2003a). In the context of these task domains, the top-down functioning of these limbic and cortical structures appears to be supramodal and responsive to target self-relevant features; but more generally, these structures are sensitive to the biased informational cues that convey implications for one’s own survival, well-being, and goal potentials (e.g. to reproduce).
Table 1.
Examples of experimental tasks that converge on (a.) feature-based (ventral MPFC—ACC—subcortical) and (b.) introspective (dorsal—ventral MPFC, dorsorostral ACC and PCC) appraisal of self-relevance
| (a.) Biased stimulus features that commonly evoke self-relevance (1—3) |
|
|
| 1. Different colored squares were rapidly presented on a screen, to which participants responded by squeezing a pneumatic bulb every time they saw a randomly interspersed green or blue target square. After responding to a target, participants saw an image of a coin with a monetary value superimposed (varying in magnitude across blocks). Ventral MPFC response was modulated by polar magnitudes of gain and loss outcome, whereas limbic and paralimbic structures were modulated by absolute presence of gain [9]. |
| 2. Affectively valent facial stimuli (Ekman’s series) were presented center-screen with bars in the left and right periphery that varied in respective orientation, followed by a fixation phase in which the face and bars were masked. In attended trials, participants were to decide whether the faces were male or female, and in unattended trials, whether the bars were of similar orientation. The attention to face task evoked ventral MPFC, paralimbic, and limbic substrata [13]. |
| 3. Participants performed three attentional tasks (attend to stimulus unpleasantness, stimulus location, and a control) at two different laser intensity levels (noxious and innocuous). During the ‘attention to unpleasantness’ task participants were instructed to selectively attend to the unpleasantness of laser pulses and provide a pain rating. Ventral and orbital MPFC, paralimbic, and limbic responses were observed during attention to pain magnitude [16]. |
|
|
| (b.) Self-referential tasks that commonly evoke introspective self-relevance (4—6) |
|
|
| 4. Participants performed two appraisal tasks (internally versus externally cued decisions) while viewing negative, positive, and neutral valence pictures from the IAPS. For the internally cued appraisal task, participants were to appraise how the picture made them feel (pleasant, unpleasant, or uncertain/neutral), which evoked a dorsal MPFC—dorsorostral ACC—PCC response [56]. |
| 5. Participants performed three appraisal tasks (self preference, significant other preference, and a control) while viewing names of different foods. For both the self-appraisal task, participants were asked to read the food name and decide whether or not they themselves like to eat that food, which evoked dorsal MPFC—dorsorostral ACC—PCC response (as did the significant other appraisal task) [59]. |
| 6. Participants performed three appraisal tasks (self-preference, subjective, and a control) while matching one of two distinctly colored reference squares against a third target colored square. For the self-preference task, participants were asked to decide which color of the two reference squares they preferred in combination with the color of the target square, which evoked a dorsal—ventral MPFC—dorsorostral ACC—PCC response [60]. |
Second, emergent branches of ACS research are starting to converge on a distinct ASR system with experiments using self-relevant tasks that impose an explicitly self-referential contingency, where participants appraise how the stimulus makes them feel, or, whether the stimulus relates to some internal mental content (see Table 1 for specific examples of task instructions). Despite using comparable stimuli, the contextual shift to one’s self as the referent of appraisal consistently evokes distinct neural structures inclusive of the ventral and dorsal MPFC, dorsorostral ACC, and posterior cingulate (PCC). Observations of this neural response pattern to such self-referential ‘modes’ have increased in close proportion with the burgeoning studies that compose this research domain. As such, these cortical midline structures (Northoff and Bermpohl 2004) appear to mediate introspective processes associated with task-induced ASR. We argue that these structures constitute a distinct albeit highly integrated component of this supramodal system, the collective functioning of which enables continuous access to one’s mental content (e.g. thoughts, memories). Recent neuroimaging research examining stimulus-independent self-referential processing, as well as the so-called resting state, has provided additional compelling support for this distinction. Therefore, in characterizing the integrative supramodal systems underlying ASR, we suggest two potentially separable sub-systems that together enable representation of sensory (e.g. exteroceptive and interoceptive) and introspective mental phenomena.
Supramodality and ASR
Ventral MPFC—ACC—subcortical system
Across a diverse array of ACS neuroimaging task domains, those requiring appraisal of biased (e.g. affective or arousing) stimulus features that convey general implications for survival or well being consistently identify activation in the ventral MPFC and ACC, Amg, striatum (ventral and dorsal), and insula. Specifically, process overlap in this sub-system is evident: in studies of reward, where the vMPFC—insula—caudate—Nacc network has been shown to differentially respond to anticipation versus outcome of financially gainful stimuli (Knutson, et al. 2003; Knutson, et al. 2005), as well as to the absolute presence (with additional Amg response) versus specific values of reward (Elliott, et al. 2003); in studies requiring appraisal of affective stimulus content, where the vMPFC—vACC—insula—Amg was co-active during passive viewing of highly aversive stimuli (Nitschke, et al. 2006), the vMPFC—vACC—caudate—Amg during passive viewing of surprised faces preceded by affectively valent sentences (Kim, et al. 2004), the vMPFC—Nacc during schematic emotional processing of valent sentences (Schaefer, et al. 2003) and the vMPFC—insula—caudate—Amg during appraisal of fearful faces in high versus low attention conditions (Pessoa, et al. 2002; Pessoa, et al. 2005); in studies of pain, where the vMPFC—vACC—insula—Amg was co-active during perceived controllability of nociceptive thermal stimuli (Salomons, et al. 2004) and appraisal of differential laser pulse magnitudes (Bornhovd, et al. 2002; Kulkarni, et al. 2005); and finally in a study of fear conditioning, where the vMPFC—vACC—Insula—caudate—Amg was co-active during appraisal of stimuli in the presence and absence of fear-provoking reinforcement (mild shock) (Phelps, et al. 2004) and the vMPFC—Amg during reversal of a learned fear response (aversive noise) (Morris and Dolan 2004). See Figure 1.
Figure 1.
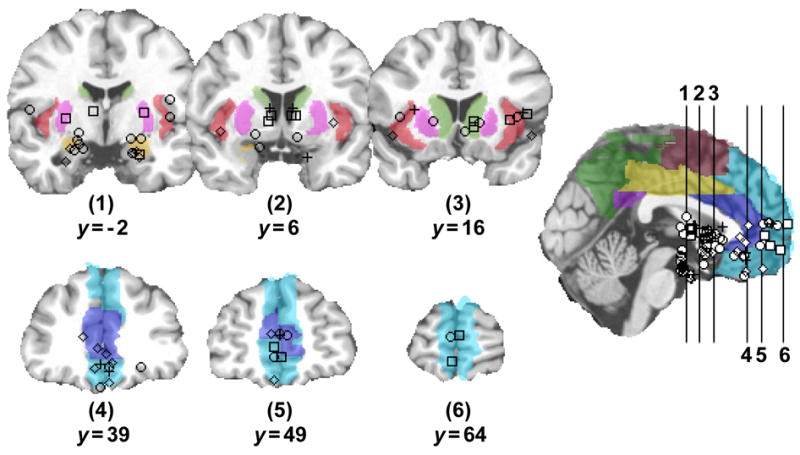
Loci of statistically significant activation (as reported by individual papers) from multiple neuroimaging task domains that utilize stimuli with a self-relevant feature. Squares = reward appraisal tasks (Elliott, et al. 2003; Knutson, et al. 2003; Knutson, et al. 2005); Circles = tasks requiring emotional appraisal of affective stimulus content (Kim, et al. 2004; Nitschke, et al. 2006; Pessoa, et al. 2002; Pessoa, et al. 2005; Schaefer, et al. 2003); Diamonds = pain appraisal tasks (Bornhovd, et al. 2002; Kulkarni, et al. 2005; Salomons, et al. 2004); Crosses = fear conditioning tasks (Morris and Dolan 2004; Phelps, et al. 2004). The loci are displayed on colored anatomical volumes of interest (AVOIs), which were selectively derived from an anatomical parcellation of the MNI single subject T1 volume (Tzourio-Mazoyer, et al. 2002). Green = caudate nucleus/accumbens; orange = amygdalae; pink = putamen; red = insula; blue = anterior cingulate; cyan = dorsal—ventral medial prefrontal cortex; yellow = mid-cingulate; purple = posterior cingulate; green = precuneus/parietal lobule; brown = supplementary motor area. The structural underlay was derived from the single subject high resolution T1 volume provided by the Montreal Neurological Institute (MNI) (Collins, et al. 1998), shown in coronal orientation at slices 1—7 with locations in the anterior—posterior plane provided, and in sagittal orientation at the midline with both coronal slice line and collated activation loci overlays. Representative activation coordinates originally provided in Talairach atlas space (Talairach and Tournoux 1988) were converted to MNI space using the ‘tal2mni’ transformation algorithm (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml). The left hemisphere is on viewer’s left side.
Given the overlap of ventral MPFC findings spanning the above ACS research, despite qualitatively different stimulus types and methods of induction, we infer that this region is indeed capable of supramodal processing, and, that the central function of this processing is to detect self-relevant information. Northoff and Bermpohl (2004) have reasoned that ventral MPFC response (identified in that review as inclusive of both orbitofrontal and inferior ventromedial BA 10,11, and 12) is commonly observed during affective processing due to the inherent self-relevance of emotional stimuli. Further, those authors posit that experimental stimuli fall along a continuum roughly divisible as self and non-self relevant, where affectively laden stimuli fall along the prior and non-referential semantic stimuli (e.g. pictures of neutral nameable objects) along the latter distribution. Separate research using the Iowa Gambling Task (Bechara, et al. 2003; Bechara, et al. 1999; Bechara, et al. 1997) has demonstrated that lesions to the ventral MPFC result in decreased aptitude to model the self-relevant feature of stimuli—increasing risk associated with certain card choices fails to invoke behavioral changes (gambling strategy) in patient cohorts. Such patients appear unable to ‘catch on’ when decision outcomes become undesirable despite accurately self-reporting an understanding of the optimal playing strategy to avoid monetary loss. Damasio and colleagues suggest that the ventral MPFC mediates a form of pre-attentive processing that draws upon contextually relevant past experiential information to implicitly bias conscious appraisal of stimuli (mediated by the dorsal MPFC, ACC, and PCC; see next section). The convergence of the above ACS task domains (e.g. reward, fear, expressive faces/words, pain) on ventral MPFC response provides emerging consensus that this region is sensitive to the prepotent, or biasing, self-relevant features common to these tasks. In this way, we argue that signaling within the ventral cortical-subcortical system, as a whole, is characterized by an ongoing mode of self-relevance detection that orients extensive brain systems through a cascade of ‘silent’ information processing when self-relevance is detected, anticipated, or suspected. In contrast to our operationalization of salience, this top-down processing is likely necessary but not sufficient to re-direct attention resources. Therefore, we attribute the role of the ventral cortical-subcortical system in ASR primarily to the localization and potentiation of information with which consequent appraisals of self-relevance may be formed, rather than in the generation of conscious appraisal.
The functional contributions of the ventral MPFC and subcortical structures to self-relevance detection are difficult to distinguish above and beyond their relative levels of processing complexity and integration. Based on observations from focal lesion patients, Damasio and Bechara (2003) suggest that appraisal and decision-making rely on distinct levels of processing complexity mediated primarily by the amygdala and ventral MPFC. Specifically, in the presence of a salient (e.g. unexpected sensory) stimulus, the amygdala forms an affective tag and generates a basic endogenous percept (e.g. skin conductance response), whereas the ventral MPFC models this information in a more complex fashion, drawing upon large amounts of input from other limbic, paralimbic, sensory, and memory structures. In a review of animal, lesion, and neuroimaging research, Phillips and colleagues (2003a) elaborate upon candidate structural components of this emotional appraisal model, highlighting evidence that identification of affective significance and production of emotional response biases are mediated by integrative processing within the Amg, brainstem nuclei, ventral striatum, insula, as well as ventral ACC and MPFC. However, taken together these models compel the question—what are the functional contributions of the aforementioned subcortical regions to facilitating a self-relevance detection mode?
In accordance with prior systems level approaches to understanding limbic function (LeDoux 2000; Morgane, et al. 2005), the present framework favors the notion that the amygdalae and striatum (inclusive of the nucleus accumbens) process information as a distributed integrative system, and, that information processing in this system is supramodal. Evidence from lesion and neuroimaging studies suggests that the primary functions of amygdalar and striatal processes are not in processing self-relevant task content per se, but rather in discrimination of and neuromodulative response to specific biasing features of the tasks. These subcortical regions likely facilitate physiological orienting in response to detection of self-relevant phenomena. For instance, Anderson and Phelps (2001) demonstrated that among patients with unilaterally and bilaterally ablated amygdala, perceptual awareness of transiently presented affective stimuli was attenuated in an attentional blink task, whereas the patient’s post-task (vMPFC mediated) capacity to detect the valence and arousal evoked by the same stimuli remained intact. Recent neuroimaging studies have also observed signal change in the amygdala during affective compared to neutral stimulus presentations, where the response to affective stimuli occurred irrespective of positive and negative valence manipulations (Hamann and Mao 2002; Liberzon, et al. 2003; Winston, et al. 2005). Findings such as these have led researchers to hypothesize that amygdalar information processing is coarse—distinguishing significant stimuli from the mundane (Anderson and Phelps 2001)—and that such processing primarily affects neuromodulation of sensory, autonomic, somatic, and endocrine systems to facilitate heightened vigilance of immediately or potentially (e.g. ambiguous social signals) self-relevant environmental cues (Davis and Whalen 2001; Whalen, et al. 1998; Whalen, et al. 2002). Similarly, research has evidenced response in the Nacc to salient non-rewarding (unexpected, arousing) stimuli (Zink, et al. 2003), contrary to the common attribution that this region is selective to reward processing. A follow-up study (Zink, et al. 2004), using rewarding stimuli but controlling for the level of salience, showed striatal (Nacc and Caudate) response to salient (performance dependent) as opposed to non-salient (passive receipt) rewarding stimuli. Taken together, these data fit well with the presently held argument that distinct contributory supramodal processes collectively give rise to self-relevance. The amygdalae and striatum appear sensitive to a broad range of tasks, all of which convey self-relevance as a function of inherent task features.
Paralimbic structures, inclusive of the anterior insula and ventral ACC, may further develop ASR by generating more complex ‘feeling’ representations based on sensory and physiological orienting mediated by limbic input and brainstem nuclei. As with the limbic system, though, the primary functions of these paralimbic regions are often confined within a narrow range of task domains. For instance, the proposed roles for the anterior insula as a ‘pain center’ or aversion substrate are somewhat exclusive of newer data showing that the insula is active during feelings of maternal attachment (Bartels and Zeki 2004), fear association (Phelps, et al. 2004), reward (Elliott, et al. 2003), empathy toward pain afflicted others (Singer, et al. 2004), and across affective task domains covering the emotional spectrum (Phan, et al. 2002). Insofar as the bilateral insula appears intimately involved with feeling states (Critchley, et al. 2004; Phan, et al. 2004; Phan, et al. 2002; Salomons, et al. 2004; Wager, et al. 2004), independent of these task-domains, it is inferred that this region (and this process) is supramodal. The ventral ACC is consistently responsive in neuroimaging studies examining induction of arousing emotional stimuli, irrespective of valence (Craig 2005; Critchley, et al. 2004; Phan, et al. 2002). With respect to a convergent supramodal process resident to the ventral ACC, this region has been implicated in transforming feeling states into motivational or anticipatory behavioral response (Craig 2005; Devinsky, et al. 1995). Furthermore, ventral ACC activations are often observed in conjunction with the anterior insula (Craig 2005; Phan, et al. 2002). As with the limbic system, the anterior insula and ventral ACC are also viewed within this framework to interact as a distributed integrative sub-system, though the paralimbic system appears to mediate between physiological orienting at the limbic level and self-relevant information processing at the cortical level.
We argue that the net process contributions of these limbic and paralimbic structures to ASR primarily affect a bodily orienting mechanism in response to ventral MPFC mediated self relevance detection. Contrary to the bottom-up propagation of salient information, which is classified here as any sensory information sufficient to re-direct attention, the vMPFC—ACC—subcortical system outlined above is considered to function in a top-down regulatory but reciprocal manner. In this way, salient sensory information may become self-relevant if such content is detected in the ventral MPFC, yet self-relevant sensory information need not be salient in order to instantiate this system (i.e. ASR may function at attentional sub-threshold). Detection of self-relevant information thus influences differential response biasing of sensory sensitivity, arousal, and feeling states, then back-propagates through ventral ACC and MPFC loci to influence neuromodulation of motivation and attention systems. As a result, detection of self-relevance from exteroceptive or interoceptive inputs may instantiate a cascade of subcortical information processing that orients one to an increased response potential, yet reintegration of this information at ventral ACC and MPFC loci may in turn prevent or diminish redirection of attention based on prior experience (e.g. through integration with contextually appropriate memory traces, one’s experiential history). Hence, within this subcortical—cortical ASR system, different task domains such as reward or fear may evoke the same response patterns, yet this system may also respond differently to the same task domain (given the initial level of stimulus self-relevance to a particular individual, or group of individuals; or, given changes in self-relevance during phasic stimulus exposures). This latter distinction may account for the variability of findings occasionally observed across neuroimaging studies examining the same appraisal-based task domain (with comparable stimulus design), though measures have been taken, quite literally, to assess this variability (Phan, et al. 2003; Phan, et al. 2004). A further source of variability, one that can profoundly affect the observed response in this system, is the relative level of introspection (e.g. self-referential thoughts, memories) accompanying task-induced extero- and interoception.
Dorsal—ventral MPFC, dorsorostral ACC and PCC
Affective, cognitive, and social (ACS) neuroimaging studies investigating introspective appraisal of salient stimuli, e.g. where one’s self is the explicit referent, consistently observe MPFC (extending along the dorsal—ventral axis) and PCC activity across a range of task domains. Specifically, this response pattern has been observed during self-appraisal of one’s own personality traits (Craik 1999; Johnson, et al. 2002; Kelley, et al. 2002; Schmitz and Johnson 2006; Schmitz, et al. 2004), as well as one’s opinions (Zysset, et al. 2002; Zysset, et al. 2003), personal morals (Greene, et al. 2004; Greene, et al. 2001; Moll, et al. 2002; Moll, et al. 2001) attitudes (Cunningham, et al. 2004), and perceptions of aesthetic beauty (Jacobsen, et al. 2006), during introspective appraisal of affective pictorial stimuli (Gusnard, et al. 2001; Ochsner, et al. 2005), during simulation of first person perspectives in a 3-dimensional virtual environment (Vogeley, et al. 2004), and during appraisal of one’s own preference between food choices (Seger, et al. 2004) as well as different color combinations (Johnson, et al. 2005). See Figure 2.
Figure 2.
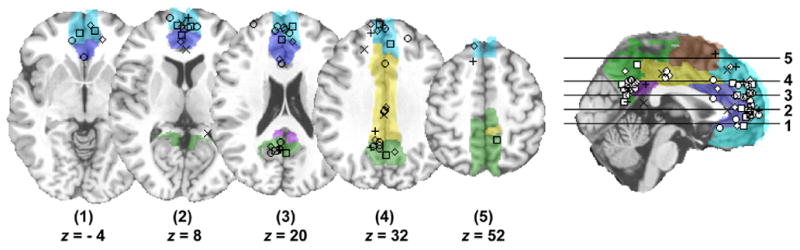
Loci of statistically significant activation (as reported by individual papers) resulting from multiple neuroimaging task domains that require self-referential introspection of stimuli. Squares = appraisal of one’s own personality traits (Craik 1999; Johnson, et al. 2002; Kelley, et al. 2002; Schmitz and Johnson 2006; Schmitz, et al. 2004); Circles = appraisal of personal morals (Greene, et al. 2004; Greene, et al. 2001; Moll, et al. 2001), opinions (Zysset, et al. 2002; Zysset, et al. 2003), attitudes (Cunningham, et al. 2004), and aesthetics (Jacobsen, et al. 2006); Diamonds = personal reaction to affective stimulus content (Gusnard, et al. 2001; Moll, et al. 2002; Ochsner, et al. 2005); Exes = appraisal of one’s own visuospatial perspective (Vogeley, et al. 2004); Crosses = appraisal of personal preferences (Johnson, et al. 2005; Seger, et al. 2004). The loci are displayed on colored AVOIs and the MNI structural underlay (shown in axial orientation at slices 1—5 with locations in the superior—inferior plane provided, and in sagittal orientation at the midline with both axial slice line and collated activation loci overlays). Graphical rendering methods were the same as those in Figure 1.
Again, these highly overlapping response patterns across a variety of task domains and stimulus types implicate an inherent supramodal processing capacity, sensitive primarily to self-relevant task features. However, these studies also have in common a task requirement that constrains the process (and neural pathway) through which ASR is engendered, specifically the criterion that stimulus-cued appraisals evoke introspection (e.g. memories, thoughts; see Table 1 for a contrast of instructional examples). As such, the dorsal—ventral MPFC and PCC may compose a distinct (albeit highly integrated) component of this ASR system, activated when information about one’s mental content is necessarily instantiated as a function of the task appraisal criteria.
This systemic distinction is especially in line with current theories of PCC function. In memory research, this region has been implicated in retrieval of episodic information (Henson, et al. 1999; Wagner, et al. 2005; Wheeler and Buckner 2004), particularly that which is personally significant (Maddock 1999; Piefke, et al. 2003; Shah, et al. 2001). Observations of PCC activation have also resulted from paradigms requiring recall and representation of complex visuospatial information (Burgess, et al. 2001; Vogeley, et al. 2004), spurring hypotheses that the PCC is involved in ‘egocentric’ modeling of spatial relativity as well as re-experiencing of past events. As a function of these multiple putative roles in autobiographical memory and spatial representation, some have questioned whether the PCC may compose distinct subregions (Nielsen, et al. 2005; Vogt, et al. 2003). Indeed, a recent combined cytological and in vivo functional connectivity (FDG PET) study suggests that these related processes are cytoarchitectonically and functionally differentiable to dorsal and ventral PCC substructures (Vogt, et al. 2006). The dorsal PCC was implicated in a visuospatial and orientation network inclusive of the dorsal ACC and premotor areas, whereas the ventral PCC shared connections within an orbital and ventral MPFC network implicated in assessing self-relevant content from visual information. Furthermore, the laterally adjacent retrosplenial cortices were found to share strong reciprocal coupling with the dorsal PCC, suggestive of a network involved in complex visuospatial memory. With regard to the diffuse PCC findings observed in the aforementioned experimental tasks requiring self-referential appraisal, we infer that introspective ASR subsumes many of these interrelated PCC subprocesses. The highly integrated visuospatial, mnemonic, and evaluative functions of these proximal medial parietal substructures are likely central to introspective experiences, as well as moment-to-moment and retrograde self-monitoring of one’s own person within a phenomenal context.
Theoretical distinctions between posterior and anterior cingulate function have implicated the latter region in executive control processes such as selective attention (Bush, et al. 2000; Vogt, et al. 1992). Given the fairly consistent observation of dorsorostral ACC response spanning the studies reviewed in this section, this region may contribute effortful regulation of and attention to introspective information evoked by self-relevant stimuli. Indeed, Lane et al. (1997) observed signal increases in rostral ACC loci when participants were instructed to selectively attend to their subjective emotional responses toward affective pictorial stimuli. Gusnard et al. (2001) replicated this finding utilizing a similar subjective appraisal paradigm, demonstrating concomitant PCC activation. Thus, the dorsorostral ACC may serve as an attention-allocating mechanism during ASR, perhaps resolving introspective mental content with task-induced extero/interoception.
It should be noted here that the spatial precision of activation clusters within the medial frontal cortex, primarily between rostral ACC and dorsal MPFC loci, is somewhat coarse in many of the studies reviewed in this section (Gusnard, et al. 2001; Johnson, et al. 2002; Johnson, et al. 2005; Lane, et al. 1997; Nitschke, et al. 2006; Schmitz and Johnson 2006; Vogeley, et al. 2004). This is due in part to the inherently large-scale medial frontal neural signatures evoked by introspective ASR, which obscure highly integrative but likely distinguishable subprocesses mediated by cingulate and cortical substrates. In contrast to the attention allocating mechanism of the dorsorostral ACC, the dorsal anterior MPFC (extending along the midline to ventral regions) is strongly implicated in evaluative processing (inclusive of detection; see prior section) of self-relevant mental content, whether to facilitate a decision about an explicitly self-referential stimulus (e.g. studies reviewed in Figure 2), guide contextually appropriate behavior through affective response regulation (Gusnard, et al. 2001; Phillips, et al. 2003a), form socially relevant impressions about others (Cunningham, et al. 2003; Mitchell, et al. 2005), or to generate inferences about the mental content of others—Theory of Mind (ToM) (Frith and Frith 1999; Frith and Frith 2003). This distinction between anterior MPFC and dorsorostral ACC function is in line with inferences drawn from another recent meta-analysis on social cognition (Amodio and Frith 2006), wherein the prior structure was implicated in self-knowledge, mentalizing, and person perception (i.e. evaluative processes), and the latter in action monitoring (i.e. selective attention). Though many of the aforementioned studies observe concomitant dorsorostral cingulate activity, the overall consistency of dorsal MPFC response provides convergent evidence that this region is engaged by evaluation of self-relevance, particularly social self-relevance, which may compose one’s mental content in the presence or absence (D’Argembeau, et al. 2005; Kjaer, et al. 2002) of self-relevant stimuli. In the context of ASR, such evaluative processing is considered a distinct component of introspection.
Whereas the PCC, rostral ACC, and MPFC (extending along the dorsal—ventral axis) appear to subserve distinct contributory processes during introspection, perhaps even dissociable by specific task manipulation, mounting evidence from research on the so-called ‘resting state’ suggests that these regions are intrinsically coupled with one another physiologically. Studies examining participants in states of quiet repose (e.g. awake, lying still, eyes open/closed) have demonstrated high levels of baseline signal, with BOLD contrast (Greicius and Menon 2004; Lustig, et al. 2003) and metabolic assays of glucose uptake and blood flow (FDG and O15 PET) (Gusnard, et al. 2001), primarily in the ventral—dorsal MPFC, ACC, and PCC. As a result of these observations, it has been inferred that these structures compose a network characterized by a default mode (Gusnard, et al. 2001) of high level baseline activity, and further, that this baseline is in close proportion with the brain’s continuous resting oxygen extraction (OEF) level. Though this physiological profile of activity is distinct from that observed during task induced activity (the OEF decreases during transient oxygen increases; BOLD) (Gusnard, et al. 2001), the psychological profile of the cortical midline at rest remains undetermined. Well reasoned, but as of yet untested hypotheses suggest that the default mode encompasses processes similar to the presently held notions of introspection (Fransson 2005; Greicius, et al. 2004; Gusnard, et al. 2001), though this mode presumably occurs without overt stimulus cueing or a volitional component (e.g. task demand). However, the mere absence of experimenter controlled stimuli and task are insufficient as controls against context-specific cues for introspective processing. Neuroimaging paradigms permitting the freedom of waking rest likely free attentional resources to explore the experimental setting of the scanner (e.g. the bodily self-monitoring necessary to keep still and comply with the scanning procedure) and the very ‘act’ of being scanned (e.g. self-evaluative awareness of being observed or of what your brain is expected to be doing), both of which represent complex and highly self-referential stimuli conducive to introspective ASR. Thus, introspective processes evoked by self-referential tasks (mediated by MPFC and PCC) may represent the very same processes commonly attributed to the default mode. A key question, though difficult to address at present, is how the resting brain state observed whilst participants lie in the scanner compares to that which would be observed as they lie awake in the comfort and familiarity of their own bed.
Further neuroanatomical considerations
Taken separately, the supramodal integration of subcortical and cortical systems supporting ASR follow from what is known of underlying axonal connectivity between MPFC and distal brain regions in primates and humans. Tracing studies of macaques indicate that the orbital and ventral MPFC receive robust input from inferior temporal cortices, which convey memory-enriched visual information about the global environment (Barbas 2000; Desimone and Gross 1979). Additionally, the orbital MPFC receives dense projections from every other sensory modal cortical region (Barbas 2000) as well as from the amygdala, insula, and ventral anterior cingulate areas (Morecraft, et al. 1992), further implicating the primacy of these regions in the continuous pre-attentive filtering of environmental and bodily information for self-relevant content. In contrast, the dorsomedial and mid-dorsolateral PFC are highly connected in both humans and primates to regions associated with visuospatial and memory processes, such as the PCC and retrosplenial cortices, as well as areas of the superior temporal sulcus (STS) (Petrides and Pandya 1999). In humans, this dorsal midline pathway seems physiologically well suited for internal recollection (PCC, RSC, STS) and evaluation or manipulation (dorsal MPFC) of self-relevant information, or more generally, introspection. Taken together, the dorsal—ventral axis of the MPFC also share dense reciprocal connections in humans and primates (Barbas 1988; Barbas and Pandya 1989), implicating this region as an ideal neuroanatomic (due to intrinsic connectivity) substrate for binding introspective processing with ongoing awareness of one’s environmental content.
Implications and conclusions
Given the present conception of two supramodal sub-systems, consisting of a ventral MPFC—ACC—subcortical system that orients pre-attentive biasing information (e.g. anticipatory or mnemonic) to salient or explicitly self-relevant phenomena and a dorsal MPFC—cortical—subcortical system that mediates self-referential introspection (.g. self-reflection, evaluation, recollection) of such phenomena, a necessary and unresolved questions remains as to how these processes integrate to enable goal-directed behaviors (e.g. decisions about one’s preference toward a self-relevant stimulus). Previous neuroimaging research comparing passive reactions versus explicitly cognitive decisions to affectively laden stimuli suggest mutually suppressive systemic interactions, wherein vMPFC and limbic activity seems dominant in the prior task context, and dorsomedial and dorsolateral PFC activity in the latter (Drevets and Raichle 1998; Mayberg, et al. 1999). In a recent study that examined psychophysiological interactions in both the dorsal and ventral MPFC during self-appraisal decisions, a network differentiation was observed between a vMPFC—paralimbic—limbic ‘affective’ pathway and a dMPFC—cortical—hippocampal ‘cognitive’ pathway (Schmitz and Johnson 2006). Furthermore, these extended pathways were not observed in the main effect of task, wherein activation was contained exclusively in cortical midline structures inclusive of medial parietal and ACC regions, suggestive of introspective ASR. We interpret these data as indicative that the functional contributions of the dorsal MPFC and ACC to ASR are indeed distinct from those of limbic and paralimbic substrata. Further research is required to examine the supramodal processing capacity within the brain regions highlighted in this review, and, to verify that self-relevance is indeed the unitary task feature driving the observed overlap of regional findings. Promising new multivariate techniques (Caplan, et al. 2006; McKeown and Hanlon 2004) may be helpful in differentiating components of the system that exhibit simple covariance from more crucial elements of the system that may exhibit task dependent effective connectivity.
Of the overlapping findings reviewed in the first two sections covering ASR systems (see also Figures 1 and 2), a few source studies observed additional activation loci suggestive of processes described in the opposing section. As our primary objective was to provide a framework based on large-scale systematic patterns of brain activity spanning seemingly disparate lines of neuroimaging research, studies were divided into their respective sections based on the task context in which self-relevant stimuli were appraised (i.e. feature-based or introspective) and on resultant activation patterns that indicated strongly preferential engagement of structures within either of the proposed systems. However, both feature-based and introspective appraisal of stimulus self-relevance commonly activates the ventral MPFC. During ASR, this region may facilitate reciprocal updating of self-relevant information back-propagating from limbic and paralimbic regions with introspective self-referential information processed in more dorsally situated cortical midline regions (e.g. dMPFC, dorsorostral ACC, and PCC). Few neuroimaging studies have directly examined the differential contributions of introspective processing to tasks that require appraisal of self-relevant stimuli, yet this is readily assessable through intra- and extra-scan measures of participant’s subjective experience, as well as temporal manipulations that vary the duration at which ASR may occur (e.g. assuming that longer durations facilitate introspection).
A process component of ASR not considered within this framework, though recent research suggests a potential role, is the instantiation of neural models that learn and eventually simulate routinely executed cognitive processes. Ramnani (2006) argues that a cortical—cerebellar system is central to the development of such models, where higher order cognitive processes initially mediated in prefrontal cortical loci shift control iteratively to internal cerebellar representations of these processes, thereby sparing processing load on less efficient executive prefrontal resources. Clinical (Bastian and Thach 1995; Goodkin, et al. 1993), lesion (Krupa, et al. 1993; Lu, et al. 1998), and neuroimaging (Imamizu, et al. 2000) evidence exists for this cortical—cerebellar system in the acquisition of motor skills, which enables rapid ‘effortless’ execution and sequencing of bodily movements. However, a growing body of neuroimaging evidence indicates that internal cerebellar models may extend across a much broader range of contributory cognitive processing (Fiez 1996; Ramnani, et al. 2006), inclusive of attention to anticipated informational cues (Allen, et al. 1997), processing prediction errors for monetary reward (Ramnani, et al. 2004), and autobiographical memory (Svoboda, et al. 2006). As with highly efficient cerebellar models of motor actions, which simulate complex bodily actions from simple motor commands, so too these models may facilitate detection processes inherent to appraising self-relevant content from task-features, or, attentional shifting between one’s external environment and introspective mental content. The concept of self-relevance has yet to be been studied explicitly with functional neuroimaging of the cerebellum. At the very least the cerebellum likely has a role in the anticipation and generation of actions to stimuli appraised as self-relevant.
To date, affective, cognitive, and social neuroimaging research has focused intensely on understanding the brain processes involved in appraisal of self-relevant stimuli, and more importantly, how these processes conform to certain task domains. In an attempt to characterize common patterns of cortical—subcortical neural activation that are observed across such task domains, we have put forth a framework of brain function that centers on self-relevance as the core feature that drives stimulus appraisal and interaction. The large-scale neural systems in which these patterns of activation occur are defined as supramodal because they do not appear selective to specific methods of stimulus induction, but rather to features common to these methods, i.e. the level of prepotent or contextually imposed self-relevance. Furthermore, we distinguish between two broad lines of ACS research that involve either feature-based or introspective appraisal of self-relevance. An emerging body of data suggests that the prior classification preferentially evokes a ventral MPFC—subcortical system, the primary functions of which are detection of and orientation to self-relevant content. The latter classification encompasses research in which appraisals of self-relevance explicitly require introspection, and thus a contextual shift to a self-referential mode, which preferentially engages a dorsal—ventral MPFC--dorsorostral ACC—PCC system. As a whole, the purpose of this review and framework is to re-conceive the multiple different processes often attributed to these systems in terms of supramodal functions that more directly reflect the influences of task relevance to the self. As a species for which the complexity of societal structure and communication has only increased over the last 20,000 years, the development and integration of these systems has likely enabled a more sophisticated capacity to appraise one’s own behaviors, those of conspecifics, and those which reflect the dynamic feedback of social interaction. In turn, the gradual development of these appraisal systems may have facilitated the more sophisticated representations of self that differentiate humans from highly related hominoid genera.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health R01 AG021155 and R01 MH65723 and a grant from the Department of Veterans Affairs to SCJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275(5308):1940–3. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–9. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic organization of basoventral and mediodorsal visual recipient prefrontal regions in the rhesus monkey. J Comp Neurol. 1988;276(3):313–42. doi: 10.1002/cne.902760302. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52(5):319–30. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286(3):353–75. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bastian AJ, Thach WT. Cerebellar outflow lesions: a comparison of movement deficits resulting from lesions at the levels of the cerebellum and thalamus. Ann Neurol. 1995;38(6):881–92. doi: 10.1002/ana.410380608. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–69. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–81. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy [see comments] Science. 1997;275(5304):1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125(Pt 6):1326–36. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14(2):439–53. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caplan JB, Luks TL, Simpson GV, Glaholt M, McIntosh AR. Parallel networks operating across attentional deployment and motion processing: a multi-seed partial least squares fMRI study. Neuroimage. 2006;29(4):1192–202. doi: 10.1016/j.neuroimage.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, Evans AC. Design and construction of a realistic digital brain phantom. IEEE Trans Med Imaging. 1998;17(3):463–8. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9(12):566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Tara M Moroz, Morris Moscovitch, Donald T Stuss, Gordon Winocur, Endel Tulving, Shitij Kapur. In Search of the Self: A Positron Emission Tomography Study. Psychological Science. 1999;10(1):26–34. [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. J Pers Soc Psychol. 2003;85(4):639–49. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci. 2004;16(10):1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25(2):616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Res. 1979;178(2–3):363–80. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118 (Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. COGNITION & EMOTION. 1998;12(3):353–385. [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–7. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA. Cerebellar contributions to cognition. Neuron. 1996;16(1):13–5. doi: 10.1016/s0896-6273(00)80018-5. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286(5445):1692–5. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philos Trans R Soc Lond B Biol Sci. 2003;358(1431):459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkin HP, Keating JG, Martin TA, Thach WT. Preserved simple and impaired compound movement after infarction in the territory of the superior cerebellar artery. Can J Neurol Sci. 1993;20(Suppl 3):S93–104. doi: 10.1017/s0317167100048599. [DOI] [PubMed] [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44(2):389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293(5537):2105–8. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation. J Cogn Neurosci. 2004;16(9):1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13(1):15–9. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Henson R, Rugg M, Shallice T, Josephs O, Dolan R. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403(6766):192–5. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Jacobsen T, Schubotz RI, Hofel L, Cramon DY. Brain correlates of aesthetic judgment of beauty. Neuroimage. 2006;29(1):276–85. doi: 10.1016/j.neuroimage.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Kawahara-Baccus TN, Rowley HA, Alexander AL, Lee J, Davidson RJ. The cerebral response during subjective choice with and without self-reference. J Cogn Neurosci. 2005;17(12):1897–906. doi: 10.1162/089892905775008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16(10):1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. Neuroimage. 2002;17(2):1080–6. [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25(19):4806–12. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260(5110):989–91. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Youell P, Watson A, Derbyshire SW, Frackowiak RS, Friston KJ, Jones AK. Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci. 2005;21(11):3133–42. doi: 10.1111/j.1460-9568.2005.04098.x. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8(18):3969–72. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61(3):137–59. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28(4):726–33. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Lu X, Hikosaka O, Miyachi S. Role of monkey cerebellar nuclei in skill for sequential movement. J Neurophysiol. 1998;79(5):2245–54. doi: 10.1152/jn.1998.79.5.2245. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100(24):14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–6. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Hanlon CA. A post-processing/region of interest (ROI) method for discriminating patterns of activity in statistical maps of fMRI data. J Neurosci Methods. 2004;135(1–2):137–47. doi: 10.1016/j.jneumeth.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci. 2005;17(8):1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Moll J, de Oliveira-Souza R, Eslinger PJ, Bramati IE, Mourao-Miranda J, Andreiuolo PA, Pessoa L. The neural correlates of moral sensitivity: a functional magnetic resonance imaging investigation of basic and moral emotions. J Neurosci. 2002;22(7):2730–6. doi: 10.1523/JNEUROSCI.22-07-02730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, Eslinger PJ, Oliveira-Souza R. Frontopolar and anterior temporal cortex activation in a moral judgment task: preliminary functional MRI results in normal subjects. Arq Neuropsiquiatr. 2001;59(3B):657–64. doi: 10.1590/s0004-282x2001000500001. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. Journal of Comparative Neurology. 1992;323(3):341–58. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75(2):143–60. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. Neuroimage. 2004;22(1):372–80. doi: 10.1016/j.neuroimage.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Balslev D, Hansen LK. Mining the posterior cingulate: segregation between memory and pain components. Neuroimage. 2005;27(3):520–32. doi: 10.1016/j.neuroimage.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29(1):106–16. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8(3):102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage. 2006;31(1):440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99(17):11458–63. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28(1):249–55. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11(3):1011–36. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, Britton JC, Liberzon I. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biol Psychiatry. 2003;53(3):211–5. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003a;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003b;54(5):515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–68. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Johansen-Berg H, Richter MC, Pinsk MA, Andersson JL, Rudebeck P, Ciccarelli O, Richter W, Thompson AJ, et al. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from Macaque monkeys and humans. Cereb Cortex. 2006;16(6):811–8. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Elliott R, Athwal BS, Passingham RE. Prediction error for free monetary reward in the human prefrontal cortex. Neuroimage. 2004;23(3):777–86. doi: 10.1016/j.neuroimage.2004.07.028. [DOI] [PubMed] [Google Scholar]
- Salomons TV, Johnstone T, Backonja MM, Davidson RJ. Perceived controllability modulates the neural response to pain. J Neurosci. 2004;24(32):7199–203. doi: 10.1523/JNEUROSCI.1315-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Collette F, Philippot P, van der Linden M, Laureys S, Delfiore G, Degueldre C, Maquet P, Luxen A, Salmon E. Neural correlates of “hot” and “cold” emotional processing: a multilevel approach to the functional anatomy of emotion. Neuroimage. 2003;18(4):938–49. doi: 10.1016/s1053-8119(03)00009-0. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. Neuroimage. 2006;30(3):1050–8. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical Activations during judgments about the self and an other person. Neuropsychologia. 2004;42(9):1168–77. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. The neural correlates of person familiarity: A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124(Pt 4):804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain 3-Dimensional Proportional System: An Approach to Cerebral Imaging. New York: Thieme; 1988. [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink GR. Neural correlates of first-person perspective as one constituent of human self-consciousness. J Cogn Neurosci. 2004;16(5):817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18(11):3134–44. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29(2):452–66. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18(1):411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H. Functional neuroimaging studies of the amygdala in depression. Semin Clin Neuropsychiatry. 2002;7(4):234–42. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21(4):1337–49. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. J Neurosci. 2005;25(39):8903–7. doi: 10.1523/JNEUROSCI.1569-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42(3):509–17. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS. Human striatal response to salient nonrewarding stimuli. J Neurosci. 2003;23(22):8092–7. doi: 10.1523/JNEUROSCI.23-22-08092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The Anterior Frontomedian Cortex and Evaluative Judgment: An fMRI Study. Neuroimage. 2002;15(4):983–91. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Samson A, Ferstl EC, von Cramon DY. Functional specialization within the anterior medial prefrontal cortex: a functional magnetic resonance imaging study with human subjects. Neurosci Lett. 2003;335(3):183–6. doi: 10.1016/s0304-3940(02)01196-5. [DOI] [PubMed] [Google Scholar]


