Abstract
We have used the yeast two-hybrid system to isolate cDNAs encoding proteins that specifically interact with the 42-aa β-amyloid peptide (Aβ), a major constituent of senile plaques in Alzheimer’s disease. The carboxy terminus of α2-macroglobulin (α2M), a proteinase inhibitor released in response to inflammatory stimuli, was identified as a strong and specific interactor of Aβ, utilizing this system. Direct evidence for this interaction was obtained by co-immunoprecipitation of α2M with Aβ from the yeast cell, and by formation of SDS-resistant Aβ complexes in polyacrylamide gels by using synthetic Aβ and purified α2M. The association of Aβ with α2M and various purified amyloid binding proteins was assessed by employing a method measuring protein–protein interactions in liquid phase. The dissociation constant by this technique for the α2M–Aβ association using labeled purified proteins was measured (Kd = 350 nM). Electron microscopy showed that a 1:8 ratio of α2M to Aβ prevented fibril formation in solution; the same ratio to Aβ of another acute phase protein, α1-antichymotrypsin, was not active in preventing fibril formation in vitro. These results were corroborated by data obtained from an in vitro aggregation assay employing Thioflavine T. The interaction of α2M with Aβ suggests new pathway(s) for the clearance of the soluble amyloid peptide.
Senile plaques in the brain and cerebral blood vessels of patients with Alzheimer’s disease are composed primarily of the aggregated form of Aβ (1, 2). The Aβ peptide is derived post-translationally by proteolytic activity from a larger amyloid precursor protein (3–10). The mechanism for Aβ clearance or for its deposition is not known. Two proteinase inhibitors, α2-macroglobulin (α2M) and α1-antichymotrypsin (α1ACT), have been identified as being associated with senile plaques (11, 12, 13). α2M is capable of binding to and blocking the proteolytic activity of most proteinases before rapid clearance of these α2M -proteinase complexes by the low density lipoprotein receptor-related protein (LRP). Internalization and degradation of α1ACT-proteinase complexes are mediated by the serpin-enzyme complex receptor. Significantly increased levels of both α2M and α1ACT are often found in localized areas of inflammation (14, 15, 16). The full range of biological activities of α2M and α1ACT still remains to be defined.
In an effort to identify proteins that interact in vivo with Aβ and therefore might play a role in its clearance or deposition, we screened a HeLa library using the yeast two-hybrid system (17–21). One of the proteins determined to have a strong and specific interaction with Aβ was α2M. To examine the possible role of this interaction in neurotoxic amyloid fibril formation, we investigated the following: (i) the in vivo binding of Aβ to α2M in the yeast cell; (ii) the in vitro binding affinity of Aβ to α2M compared with that of Aβ to other amyloid-binding proteins; and (iii) the effect of α2M, α1ACT, and apolipoprotein (apo) J on fibril formation.
EXPERIMENTAL PROCEDURES
Bacterial and Yeast Strains.
Manipulations of bacterial strains and of DNAs were by standard methods (22, 23).
Construction of Bait and Prey Plasmids.
The bait plasmids (LexA-Aβ and LexA-C100 fusion proteins) were constructed as described (17). The bait plasmid encoding LexA-bicoid fusion protein and the prey plasmids (fusion of B42 to cDNAs from HeLa library; ref. 18) were kindly provided to us by Roger Brent (Massachusetts General Hospital, Boston). The prey fusion proteins were inducible in yeast grown on minimal medium containing 2% galactose and 1% raffinose (Gal/Raf) but not in yeast grown on 2% Glc. Western blot analyses (24) were performed to show that the bait and prey plasmids expressed the expected fusion proteins (data not presented).
Transformation of Strain with Reporter, Bait, and HeLa Library Prey Plasmids.
The selection strain was created by transforming the EGY48 yeast strain with a URA3 lacZ [β-galactosidase (β-gal)] reporter plasmid and the HIS3 bait plasmid (22) as described (17). The yeast selection strain harboring the bait and reporter plasmids was transformed with prey plasmid DNA (22), and tryptophan utilization phenotype was used for selection of transformants.
Determination of Bait–Prey Interaction.
Yeast strains containing the appropriate bait and prey plasmids were grown to OD600 = 0.5, diluted 1,000-fold, and spotted on plates containing Gal/Raf Ura− His− Trp− 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) medium or Glc Ura− His− Trp− X-Gal medium for assessing the transcriptional activation of the lacZ reporter gene. Suitably diluted cell suspensions also were spotted on Gal/Raf Ura− His− Trp− Leu− medium and Glc Ura− His− Trp− Leu− medium to assess the transcriptional activation of the leucine gene.
β-Gal Activity in Liquid Cultures of Yeast.
Cells were assayed for β-gal activity using the O-nitrophenyl-β-d-galactoside method (22).
Immunoprecipitation and Western Blotting.
Preparation of cell extracts, immunoprecipitation, and Western blotting were performed as described (17). Western analysis of the blotted proteins was performed with enhanced chemiluminescence reagents (Amersham) using the 12CA5 anti-hemagglutinin mAb (25).
Binding Studies on Polyacrylamide Gels.
Purified α2M (from human plasma) was obtained from Calbiochem. For the binding studies with purified proteins (Fig. 4), 0.5 μl of a 1 mg/ml solution of Aβ was incubated overnight with 10 μl of a 4 mg/ml solution of α2M. To confirm the Aβ binding site on α2M, 100 μl of α2M solution (4 mg/ml) was incubated with 2 ml of hydroxylamine for 4 hr at 45°C, dialyzed against distilled water, and lyophilized. The resulting fragments were incubated with Aβ peptide as described above. Electrophoresis was carried out using 4–15% SDS polyacrylamide gels. Immunoblots were developed by using the anti-Aβ antibodies 4G8 and 6E10.
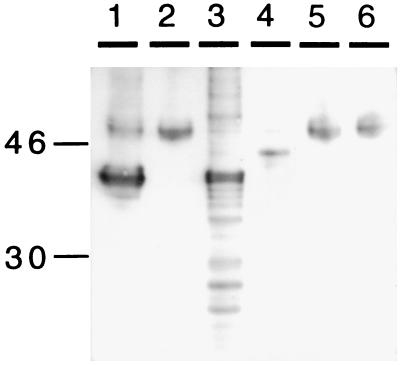
Figure 4.
Binding between synthetic Aβ 1–40 and purified human α2M. A 0.5 μl of a 1 mg/ml solution of Aβ was incubated overnight at room temperature with 10 μl of a 4 mg/ml solution of α2M. Electrophoresis was carried out using 4–15% SDS/PAGE. Immunoblots were developed by using anti-Aβ antibodies 4G8 or 6E10. Lanes: 1, Aβ alone; 2, α2M alone; 3, Aβ incubated with α2M; and 4, Aβ incubated with hydroxylamine-treated α2M. The gel is representative of a set of three independent experiments.
Determination of Kd for Interaction with Aβ.
Labeling of α2M and other amyloid binding proteins with TAG-NHS ester was performed as described in the supplier’s kit (IGEN, Gaithersburg, MD), and binding was determined using the Origen Analyzer (IGEN). Aβ-(1–40) was end-biotinylated by Peninsula Laboratories. The reaction was carried out for 2 hr at room temperature with shaking. The binding was determined at several fixed concentrations of TAG-labeled proteins (from 2 to 100 nM).
Thioflavine T Assay for Aβ Aggregation.
Aβ-(1–40) from Bachem was dissolved at a concentration of 10 mg/ml (2.5 mM) in dimethyl sulfoxide. The stock was diluted to 0.1 mg/ml (25 μM) in PBS and filtered through a 0.2-μm filter. The Aβ solution was added to a 96-well plate at 60 μl/well in triplicate along with the test proteins at concentrations ranging from 0 to 1 mM. The plates were incubated with shaking at 37°C. After 4 days, 240 μl of 20 μM Thioflavine T (in 50 mM potassium phosphate buffer, pH 6.0) was added to each well. Fluorescence was read 30 min later at Ex = 450 nm and Em = 482 nm using a Cytofluor II microplate reader (Biosearch, Bedford, MA).
Electron Microscopy.
The concentration of Aβ-(1–40) was 50 μM, and the concentration of each of the test compounds, α2M, apoJ, or α1ACT, was 6 μM. Freshly solubilized Aβ peptide was incubated in PBS, pH 7.4, alone or with test compound at 37°C for four days. Incubations were performed as in ref. 26. A 5-μl portion of the fresh or aggregated Aβ solution was applied to a 200-mesh, carbon formvar-coated copper grid. Excess fluid was blotted, and the grids were allowed to air dry. The grids were stained with 2% uranyl acetate for 5 min, rinsed with distilled water, and blotted dry. Observations were made at different magnifications on a JEOL JEM 100S electron microscope at 80 kV.
RESULTS
Interaction of Aβ with α2M.
Using the system described by Golemis and Brent (19), we created a yeast selection strain containing a hybrid gene encoding Aβ 1–42 as a bait fused in frame to the bacterial LexA DNA-binding domain that by itself has no transcriptional activation function in yeast (27). The host strain contains LEU2 and lacZ reporter genes carrying LexA operators instead of native upstream activating sequences. A strain containing the bait (LexA-Aβ) and the reporters (LEU2 and lacZ) remains inert for the expression of leucine utilization or β-gal activity unless it also contains a vector (prey) that expresses an interacting protein as a molecule fused to the B42 trans-activation domain (18). The activation-tagged cDNA-encoded prey proteins are expressed in yeast grown on galactose but not in yeast grown on Glc.
The prey plasmid library (from HeLa cells) was first screened for the ability to grow on medium devoid of leucine. Of the ≈4 × 106 colonies screened, ≈0.1% exhibited growth on this medium. Approximately 30% of these also exhibited a blue color on Gal/Raf X-Gal medium. The B42-α2M prey plasmid, when introduced into the yeast strain with LexA-Aβ bait plasmid, produced blue colonies on X-Gal medium and showed growth on minimal medium plates devoid of leucine in the presence of Gal/Raf as the carbon source but showed no blue-colored colonies and no growth in the absence of leucine with Glc (data not shown). These results indicate that the interaction between LexA-Aβ bait and B42-α2M prey was triggered by expression of the prey protein under the influence of the GAL1 promoter. When LexA-bicoid and LexA-C100 were used as baits, no interaction was observed irrespective of the carbon source used (data not shown), indicating that the interaction between LexA-Aβ and B42-α2M is specific. The cDNA for the prey protein described above corresponds to the 250 C-terminal aa of α2M (28, 29) (Fig. 1). The yeast two-hybrid screen therefore suggests a strong and specific interaction between Aβ and the carboxy-terminus of α2M.
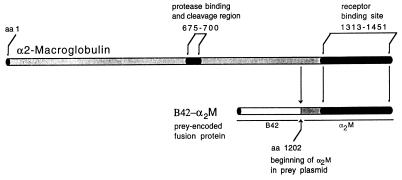
Figure 1.
Diagram of α2M monomer indicating regions important to its function. Aligned below the monomer is the fusion protein encoded by the prey plasmid B42-α2M, containing B42 activation domain followed in frame by the final 250 amino acids of the α2M C-terminus from Ser 1202 to Ala 1451. Prey plasmid sequence does not include the protease binding and cleavage regions of α2M. However, it incorporates the entire receptor binding site of α2M.
Quantitation of Interaction.
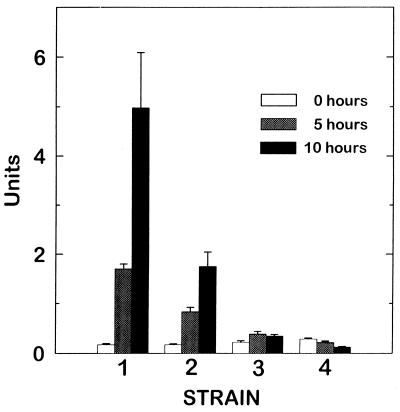
To quantitate the observed Aβ–α2M interaction, we used the O-nitrophenyl-β-d-galactoside colorimetric assay. The results presented in Fig. 2 indicate that there is significantly higher β-gal activity in the yeast strains expressing B42-α2M prey/LexA-Aβ bait (strain 1) compared with: (i) yeast cells expressing B42-α2M prey/LexA-bicoid bait (strain 2), (ii) Aβ in the bait plasmid but no prey DNA insert in the B42 plasmid (strain 3), or (iii) no prey DNA linked to B42 and no bait DNA linked to LexA (strain 4), confirming that the observed interaction between Aβ and α2M is strong and specific.
Figure 2.
O-nitrophenyl-β-d-galactoside assay to assess transcriptional activation of the lacZ reporter gene. Yeast strains were incubated in Gal/Raf complete minimal media for 0, 5, and 10 hr. Then cells were lysed, and β-gal activity was determined using the O-nitrophenyl-β-d-galactoside method. Yeast strains contained the following prey/bait plasmids: strain 1, B42-α2M/LexA-Aβ; strain 2, B42-α2M/LexA-bicoid; strain 3, B42 only/LexA-Aβ; and strain 4, B42 only/Lex A only. Numbers are representative of a set of three independent experiments.
Formation of Bait–Prey Complexes.
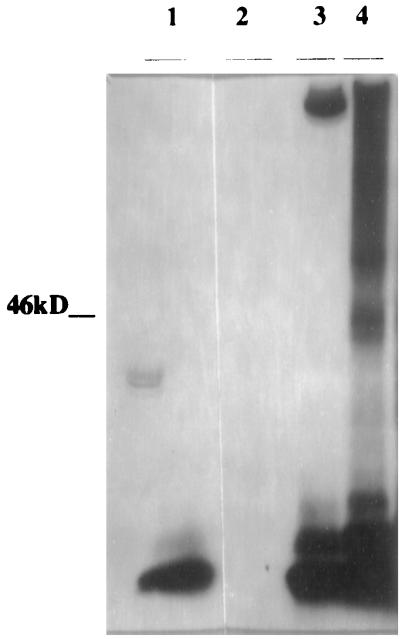
We attempted to obtain direct in vivo evidence for the interaction between B42-α2M prey and LexA-Aβ bait proteins (α2M/Aβ complex) by using anti-Aβ antibodies 4G8 and 6E10. If Aβ (bait) reacts with α2M (prey), a bait–prey complex may be coprecipitated with antibodies specific to the bait, and the prey fusion protein may be visualized as a band of 40 kDa on a Western blot using an anti-hemagglutinin antibody to this protein. Indeed, the prey-specific hemagglutinin immunoreactivity for the B42-α2M fusion protein is observed at 40 kDa from immunoprecipitated extracts obtained from cells grown in the presence of galactose (Fig. 3; lane 1) but not from those obtained from cells grown in Glc (Fig. 3; lane 2). When cell extracts were subjected directly to immunoblotting (without prior immunoprecipitation) with anti-hemagglutinin antibody, the 40-kDa band was observed from cells grown in the presence of galactose (Fig. 3; lane 3) and not from cells grown in Glc (Fig. 3; lane 4). No immunoreactive bands were observed from immonoprecipitates of cells with Aβ-LexA bait but no α2M insert in the prey plasmid (Fig. 3; lane 5) or from cells containing no inserts in the bait or prey plasmids (Fig. 3; lane 6). These results suggest an in vivo interaction between α2M and Aβ within the yeast cell.
Figure 3.
Bait-prey complexes from yeast cell extracts were immunoprecipitated with anti-Aβ mAbs 4G8 and 6E10. Immunoprecipitates were run on a 10–20% SDS/PAGE Tris-N-[tris(hydroxymethyl)methyl]glycine gel. Gels were blotted and then blots developed with an anti-hemagglutinin antibody recognizing the prey fusion protein. A band of ≈40 kDa corresponding to this protein was detected in immunoprecipitated extracts from cells grown in the presence of galactose (lane 1) but not in the presence of Glc (lane 2). The same 40-kDa band was seen in cell extracts not subjected to immunoprecipitation from cells grown in the presence of galactose (lane 3) but not in the presence of Glc (lane 4). No 40-kDa band was seen in immunoprecipitated extracts from a strain not containing prey cDNA (B42 only/LexA-Aβ) grown in Glc medium (lane 5) or in the EGY48 strain, which does not have bait or prey inserts and was grown in rich medium (lane 6). The gel is representative of a set of three independent experiments.
Binding Between Aβ and Purified α2M.
When synthetic Aβ-(1–40) peptide was mixed with α2M purified from human plasma. After SDS/PAGE and immunoblotting, in addition to the 4-kDa band for Aβ, an upshifted band corresponding to the molecular weight of α2M (≈750 kDa) was observed with mAbs (4G8 and 6E10) against Aβ (Fig. 4, lane 3). These results are in agreement with data of Du et al. (32). Furthermore, a preparation of purified α2M subjected to hydroxylamine treatment (a condition that yields an ≈43-kDa C-terminal fragment containing the receptor binding site) followed by mixing with Aβ, then SDS/PAGE and immunoblotting, yielded an ≈46-kDa band on immunoblots probed with anti-Aβ antibody (Fig. 4, lane 4), a result consistent with the binding of Aβ peptide to the C-terminal receptor binding domain of α2M. A similar Aβ-α2M complex was obtained when 125I-labeled Aβ was mixed with the hydroxylamine digestion fragment, followed by SDS/PAGE and exposure to enhanced chemiluminescence film (data not shown).
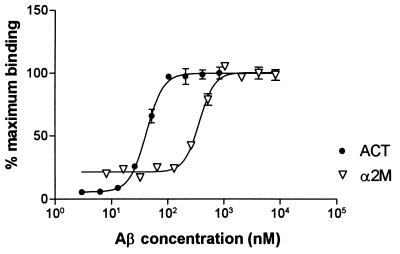
To assess the extent of the association between Aβ and α2M, we devised a method for determining the binding between two proteins in liquid phase (see Experimental Procedures). Purified native human α2M was labeled with an electrochemiluminescent reagent (N-hydroxysuccinimide ester of a ruthenium (II) tris-bipyridine chelate; TAG-NHS ester), and the amino terminus of Aβ-(1–40) peptide was labeled with biotin. Increasing concentrations of biotinylated Aβ-(1–40) were added to several fixed concentrations of TAG-labeled α2M (5.5 nM, 28 nM, and 56 nM), and the electrochemiluminescence produced by the material attached to the streptavidin-coated magnetic beads was determined. The binding between Aβ and α2M was found to be saturable (Fig. 5), specific, and reversible. The specificity was ascertained by measuring the binding of both Aβ and α2M to a number of known proteins (including BSA; data not shown). The binding between Aβ and α2M could be reversed by the addition of increasing quantities of unlabeled Aβ peptide in the reaction mixture (data not shown). A Kd of 350 nM was determined for the interaction between Aβ and α2M (Table 1) in contrast to a Kd of 0.38 nM reported by Du et al. (32) for binding between iodinated Aβ-(1–42) and α2M. These differences may be due to the fact that we use Aβ peptide labeled singly at the N-terminal end with biotin. A Kd value of 2 nM has been reported for the Aβ–apoJ interaction by Matsubara et al. (33) in contrast to the Kd value of 50 nM obtained by the liquid–phase interaction method described here. This difference may be because of the fact that Matsubara et al. (33) used immobilized peptide (apoJ incubated with Aβ 1–40 coated wells) followed by detection by using anti-apoJ antibodies to study the interaction. The relative affinities of the interactions between Aβ and other amyloid binding proteins such as transthyretin, apoE3, apoE4, apoE2, α1ACT, anti-Aβ antibody 4G8, and anti-Aβ antibody FCA3340 as determined by this method are listed in Table 1.
Figure 5.
Binding of end-biotinylated synthetic Aβ 1–40 with TAG-labeled proteins. A range of concentrations of Aβ peptide labeled with N-terminal biotin was incubated with fixed concentrations of TAG-labeled proteins at room temperature for 2 hr with shaking. The reaction mixture was then introduced into a electrochemiluminescent analyser. The intensity of chemiluminescence measured was plotted against the Aβ concentration to determine Kd (see Table 1).
Table 1.
Characteristics of TAG-labeled proteins/end-biotinylated Aβ interaction
| Protein | Kd, nM* | SEM | N |
|---|---|---|---|
| α2M† | 350 | 20 | 8 |
| Transthyretin‡ | 190 | 30 | 6 |
| ApoE3§ | 200 | 20 | 6 |
| ApoE4§ | 170 | 30 | 5 |
| ApoE2§ | 210 | 40 | 5 |
| α1-Antichymotrypsin¶ | 44 | 4 | 5 |
| ApoJ‖ | 50 | 6 | 4 |
| Antibody FCA3340** | 1.7 | 0.8 | 3 |
| Antibody 4G8‡‡ | 0.8 | 0.07 | 3 |
SEM, standard error of the mean. N, number of independent experiments.
For conditions and representative sigmoidal curves see Fig. 5.
Purified from human plasma (Calbiochem).
Recombinant protein from Dr. Y. Xie (University of Texas, College Station, TX).
Human recombinant (Calbiochem).
Purified from human plasma (Sigma).
Purified from human plasma, from J. Ghiso (New York University Medical Center, NY).
**Polyclonal antibody specific to Aβ 1–4038.
mAb against Aβ 17–28 (Senetek, Maryland Heights, MD).
Effect on in Vitro Aβ Fibril Formation.
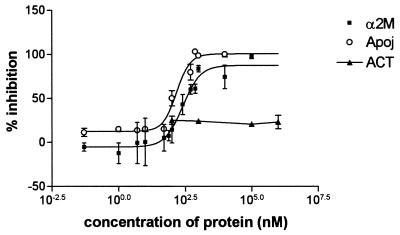
Thioflavine T interacts in some unknown way with crossed β-sheet structures common to the aggregated amyloid protein (34) (oligomers and various forms of fibrils). We measured the aggregation of Aβ peptide using the Thioflavine T binding assay similar to that described by LeVine et al. (34). Incubation of a solution containing Aβ-(1–40, 25 μM) and α2M (0–5 μM) for 4 days at 37°C demonstrated significant inhibition of the aggregation (Fig. 6), with an IC50 of 280 nM. A solution of Aβ incubated with apoJ also demonstrated inhibition of aggregation, with an IC50 of 120 nM (Fig. 6). In contrast, a solution of Aβ incubated under the same conditions with α1ACT showed no significant inhibition of aggregation of the Aβ peptide (Fig. 6). Our results point to robust anti-aggregation effects of α2M and apoJ; however the data indicate that α1ACT has no significant effect on aggregation.
Figure 6.
Thioflavine T assay for monitoring Aβ aggregation. Aβ 1–40 (25μM) was incubated at 37°C for 4 days in 1× PBS with α2M, ApoJ, and α1ACT, each at a range of concentrations. IC50 for α2M–Aβ interaction was 280 nM (SEM = 20); IC50 for ApoJ–Aβ interaction was 120 nM (SEM = 20). Data for each protein represent sets of at least three independent experiments.
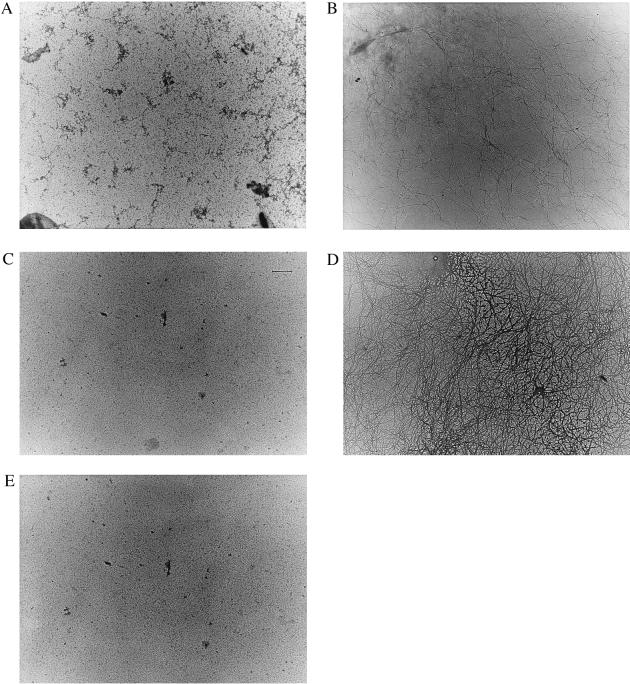
Solutions of Aβ (50 μM) in water incubated for 4 days at 37°C in the presence of 6 μM α2M (ratio ≈8:1) showed no Aβ fibril formation (Fig. 7A) in contrast to the typical fibrils formed as a result of incubating Aβ alone under similar conditions (Fig. 7B). A solution of apoJ (6 μM) incubated with Aβ under these conditions showed a decrease in fibrils formed (Fig. 7C) compared with a solution of Aβ alone. A solution of Aβ with α1ACT (6 μM) displayed significant Aβ fibril formation (Fig. 7D) under similar incubation conditions. As a control, a solution of α2M (6 μM) was incubated under the same conditions in the absence of Aβ. No fibril formation was observed in the electron micrograph for this solution (Fig. 7E) nor in solutions of apoJ alone or α1ACT alone (data not presented).
Figure 7.
Transmission electron micrographs of Aβ-interacting proteins incubated at 37°C for 4 days with or without Aβ 1-40 (50 μm in 1× PBS): (A) Aβ with 6 μM of α2M, (B) Aβ alone, (C) Aβ with 6 μM of ApoJ, (D) Aβ with 6 μM of α1ACT, and (E) α2M alone (6 μM) incubated as described above. All electron micrographs are at 15,000× magnification. Photographs are representative of at least three independent experiments.
It is important to note that Thioflavine T method measures β-pleated sheet structures, not visible fibrils; therefore the observed differences between stoichiometry of inhibition (≈1:100 ratio of Aβ to α2M in Thioflavine T assay vs. 1:8 ratio of Aβ to α2M in electron microscopy study) are not surprising. Our data suggest that α2M and apoJ are potent inhibitors of amyloid aggregation and fibril formation.
DISCUSSION
The data presented in this paper suggest a strong and specific interaction between Aβ peptide and the last 250 aa of the C-terminal region of α2M (Fig. 1). In this yeast system, no interaction was observed between the last 100 aa of amyloid precursor protein and α2M or between Drosophila bicoid protein and Aβ indicating the selectivity and specificity of Aβ–α2M interaction (data not shown). The strength of this interaction was confirmed using the β-gal colorimetric assay (Fig. 2). The protein complexes corresponding to α2M and Aβ were directly isolated by immunoprecipitation from yeast cells (Fig. 3) to demonstrate in vivo binding. The interaction between purified α2M and Aβ was confirmed on SDS-polyacrylamide gels using labeled Aβ (data not presented) or by using antibodies raised against Aβ peptide (Fig. 4). The interaction between Aβ and other amyloid-binding proteins was directly measured by utilizing an electrochemiluminescence-based liquid phase interaction assay (described in detail in Experimental Procedures section; Fig. 5 and Table 1). This interaction assay indicated that the affinity of interaction between Aβ and α2M was comparable to the affinity of interaction between Aβ and transthyretin and between Aβ and apoE2, apoE3, and apoE4. The binding between Aβ and α2M was found to be saturable (Fig. 5), reversible, and specific. Finally, we have shown that α2M inhibits the β-sheet formation (Fig. 6) and fibril-formation (Fig. 7) activities of Aβ.
The formation of amyloid fibril requires a chemically discriminating nucleation event. The kinetics of aggregation have been characterized by a delay period during which the solution remains clear followed by nucleation event that leads to a growth phase typified by viscous and turbid solution in which insoluble fibrils can be found (35). The aggregation can therefore be inhibited by influencing the nucleation event. It is therefore not surprising to find that α2M and apoJ in spite of having a 1:1 binding stoichiometry inhibit the formation of fibrils at much lower molecular abundance, presumably by decreasing the availability of amyloid monomers that can participate in nucleation events leading to β-pleated structures and fibrils. A similar stoichiometry has been reported for inhibition of Aβ peptide fibril-formation by serum amyloid P component (5:1 ratio of Aβ peptide to serum amyloid P) (36). The stoichiometries of inhibition are different between the electron microscopy analysis and the Thioflavine T assay because the former method relies on visual observation of fibrils whereas the later is a measure of β-pleated sheet structures (not all β-pleated sheet structures form visible fibrils).
The affinities of interaction between Aβ and apoJ and between Aβ and α1ACT were found to be approximately 9 to 10 times stronger compared with the affinity of interaction between Aβ and α2M (Table 1). Both α2M and apoJ strongly inhibited the formation of β-pleated sheet structures (Fig. 6) and fibril-formation (Fig. 7) by the Aβ(1–40) peptide, whereas α1ACT did not have any effect on these activities. The effect of α1ACT on Aβ fibril formation has been described in literature as both inhibition and enhancement (16, 37). The data presented in Figs. 6 and 7 clearly point to no significant effect of α1ACT on the formation of β-pleated sheets or of fibrils. Our data also indicate that the strong binding affinity of a protein to Aβ peptide is not predictive of its effects on the aggregation of the amyloid peptide.
Because the association between α2M and Aβ is strong and specific, this complex may be internalized by LRP providing for an additional mechanism of Aβ clearance. Low density lipoprotein receptor-related protein (LRP), in addition to being the native receptor for α2M, also serves as a major apoE receptor in the central nervous system. The LRP-mediated uptake of apoE/Aβ complexes may be a mechanism of Aβ clearance from the neuropil (12). The cellular uptake and subsequent degradation of amyloid precursor protein containing the first 17 amino acids of amyloid peptide also is mediated by LRP (30). Qiu et al. (31) have reported degradation of Aβ by a Ser protease-α2M complex. In that case, α2M may aid the presentation of the amyloid peptide substrate to the enzyme because this proteolytic activity is associated with the α2M-protease complex (rather than protease alone). Recently Narita et al. (36) have demonstrated that α2M/125I-Aβ complexes may be degraded by glioblastoma cells and fibroblasts via LRP. The LRP receptor may therefore play an important role in the CNS tissue by facilitating the clearance of α2M-bound Aβ peptide.
Additional experimentation will be necessary to determine the various mechanisms by which amyloid peptide and its fragments may be internalized by LRP. It has been noted that LRP and all seven known LRP ligands are associated with amyloid plaques. Because these ligands are rapidly internalized and degraded when bound to intact LRP, it has been suggested that LRP may be dysfunctional in AD (12). That is, aggregates of Aβ in senile plaques may be bound by apoE or α2M; LRP then binds but cannot internalize these complexes. Future experiments will be directed toward understanding the mechanisms by which Aβ, α2M, and apoE isoforms are internalized individually, and in combination, in various cell systems.
Acknowledgments
We thank Dr. Roger Brent (Massachusetts General Hospital, Boston) for providing the necessary vectors and yeast strains required for completion of this study. We thank Dr. Timm Jessen for help with several experimental protocols; Michael Merriman for oligonucleotide synthesis, peptide synthesis, and help with DNA sequencing; and E. Castriciones, L. Leonard, and D. Kominos for the Thioflavine T experiments. The mAbs to Aβ peptide were provided by Drs. K. S. Kim and H. Wisniewski.
ABBREVIATIONS
- Aβ
β amyloid peptide
- α2M
α2-macroglobulin
- LRP
low density lipoprotein receptor-related protein
- α1ACT
α1-antichymotrypsin
- apo
apolipoprotein
- TAG-NHS ester
N-hydroxysuccinimide ester of ruthenium(II) tris-bipyridine chelate
- β-gal
β-galactosidase
- Gal
galactose
- Raf
raffinose
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactoside
References
- 1.Masters C L, Simms G, Weinman N A, Multhaup G, McDonald B L, Beyreuther K. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glenner G G, Wong C W. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- 3.Kang J, Lamaire H G, Unterbeck A, Salbaum J M, Masters C L, Grzeschik K H, Multhaup G, Beyreuther K, Muller-Hill B. Nature (London) 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 4.Prelli F, Castano E, Glenner G G, Frangione B. J Neurochem. 1988;51:648–651. doi: 10.1111/j.1471-4159.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 5.Weidemann A G, Konig G, Bunke D, Fischer P, Salbaum J M, Masters C L, Beyreuther K. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- 6.Goldgaber D, Lerman M I, McBride O W, Saffiotti U, Gajdusek D C. Science. 1987;235:877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- 7.Haass C, Schlossmacher M G, Hung A Y, Vigo-Pelfrey C, Mellon A, Ostaszewski B L, Lieberburg I, Koo E H, Schenk D, Teplow D B, et al. Nature (London) 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 8.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schlossmacher M G, Whaley J, Swindlehurst C, et al. Nature (London) 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 9.Shoji M, Golde T E, Ghiso J, Cheung T T, Estus S, Shaffer L M, Cai X-D, McKay D M, Tintner R, Frangione B, et al. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 10.Busciglio J, Gabuzda D H, Matsudaira P, Yankner B A. Proc Natl Acad Sci USA. 1993;90:2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Gool D, De Strooper B, Van Leuven F, Triau E, Dom R. Neurobiol Aging. 1994;14:233–237. doi: 10.1016/0197-4580(93)90006-w. [DOI] [PubMed] [Google Scholar]
- 12.Rebeck G W, Harr S D, Strickland D K, Hyman B T. Ann Neurol. 1995;37:211–217. doi: 10.1002/ana.410370212. [DOI] [PubMed] [Google Scholar]
- 13.Goldgaber D, Schwarzman A I, Bhasin R, Gregori L, Schmechel D, Saunders A M, Roses A D, Strittmatter W J. Ann N Y Acad Sci. 1993;695:139–143. doi: 10.1111/j.1749-6632.1993.tb23042.x. [DOI] [PubMed] [Google Scholar]
- 14.Chu C T, Pizzo S V. Lab Invest. 1994;71:792–812. [PubMed] [Google Scholar]
- 15.Borth W. FASEB J. 1992;6:3345–3353. doi: 10.1096/fasebj.6.15.1281457. [DOI] [PubMed] [Google Scholar]
- 16.Aksenov M Y, Aksenova M V, Carney J M, Butterfield D A. Neurosci Lett. 1996;217:117–120. [PubMed] [Google Scholar]
- 17.Hughes S R, Goyal S, Sun J E, Gonzales-DeWhitt P, Riedel N G, Sahasrabudhe S R. Proc Natl Acad Sci USA. 1996;93:2065–2070. doi: 10.1073/pnas.93.5.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyuris J, Golemis E, Chertkov H, Brent R. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 19.Golemis E A, Brent R. Mol Cell Biol. 1992;12:3006–3014. doi: 10.1128/mcb.12.7.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma J, Ptashne M. Cell. 1988;55:443–446. doi: 10.1016/0092-8674(88)90030-x. [DOI] [PubMed] [Google Scholar]
- 21.Brent R, Ptashne M. Cell. 1985;43:729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- 22.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 24.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 25.Samson M L, Jackson-Grusby L, Brent R. Cell. 1989;57:1045–1052. doi: 10.1016/0092-8674(89)90342-5. [DOI] [PubMed] [Google Scholar]
- 26.Come J H, Fraser P E, Lansbury P T., Jr Proc Natl Acad Sci USA. 1993;90:5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brent R, Ptashne M. Nature (London) 1984;312:612–615. doi: 10.1038/312612a0. [DOI] [PubMed] [Google Scholar]
- 28.Sottrup-Jensen L, Stepanik T M, Kristensen T, Wierzbicki D M, Jones C M, Lonblad P B, Magnusson S, Petersen T E. J Biol Chem. 1984;259:8318–8327. [PubMed] [Google Scholar]
- 29.Kan C C, Solomon E, Belt K T, Chain A C, Hiorns L R, Fey G. Proc Natl Acad Sci USA. 1985;82:2282–2286. doi: 10.1073/pnas.82.8.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kounnas M Z, Moir R D, Rebeck G W, Bush A I, Argraves W S, Tanzi R, Hyman B T, Strickland D K. Cell. 1995;82:331–340. doi: 10.1016/0092-8674(95)90320-8. [DOI] [PubMed] [Google Scholar]
- 31.Qiu W Q, Borth W, Ye Z, Haass C, Teplow D B, Selkoe D J. J Biol Chem. 1996;271:8443–8451. doi: 10.1074/jbc.271.14.8443. [DOI] [PubMed] [Google Scholar]
- 32.Du Y, Ni B, Glinn M, Horn J W, Hamilton-Byrd E, Wu X, Bales K, Liu X, Little S P, Paul S M. J Neurochem. 1997;69:299–305. [PubMed] [Google Scholar]
- 33.Matsubara E, Frangione B, Ghiso J. J Biol Chem. 1995;270:7563–7567. doi: 10.1074/jbc.270.13.7563. [DOI] [PubMed] [Google Scholar]
- 34.LeVine H. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarrett J T, Lansbury P T. Biochemistry. 1992;31:12345–12352. doi: 10.1021/bi00164a008. [DOI] [PubMed] [Google Scholar]
- 36.Janciauskiene S, Garcia de Frutos P, Carlemalm E, Dahlback B, Eriksson S. J Biol Chem. 1995;270:26041–26044. doi: 10.1074/jbc.270.44.26041. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Yee A, Brewer H B, Jr, Das S, Potter H. Nature (London) 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 38.Narita M, Holtzman D M, Schwartz A L, Bu G. J Neurochem. 1997;69:1904–1911. doi: 10.1046/j.1471-4159.1997.69051904.x. [DOI] [PubMed] [Google Scholar]
- 39.Barelli H, Lebeau A, Vizzanova J, Delaere P, Chevallier N, Drouot C, Buxbaum J D, Khorkova O, Heroux J, Sahasrabudhe S, et al. Mol Med (Oxford) 1997;3(10):695–707. [PMC free article] [PubMed] [Google Scholar]